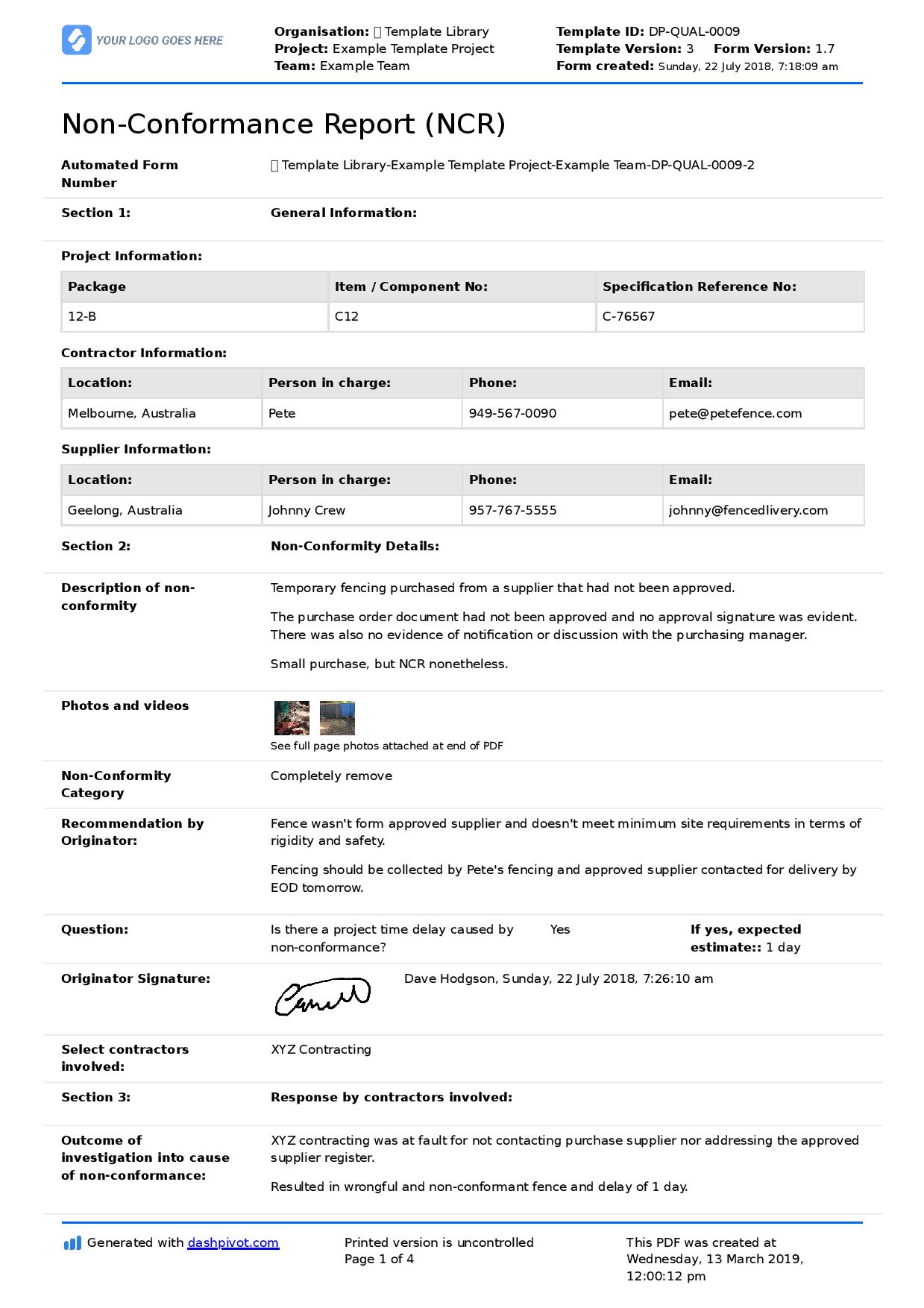
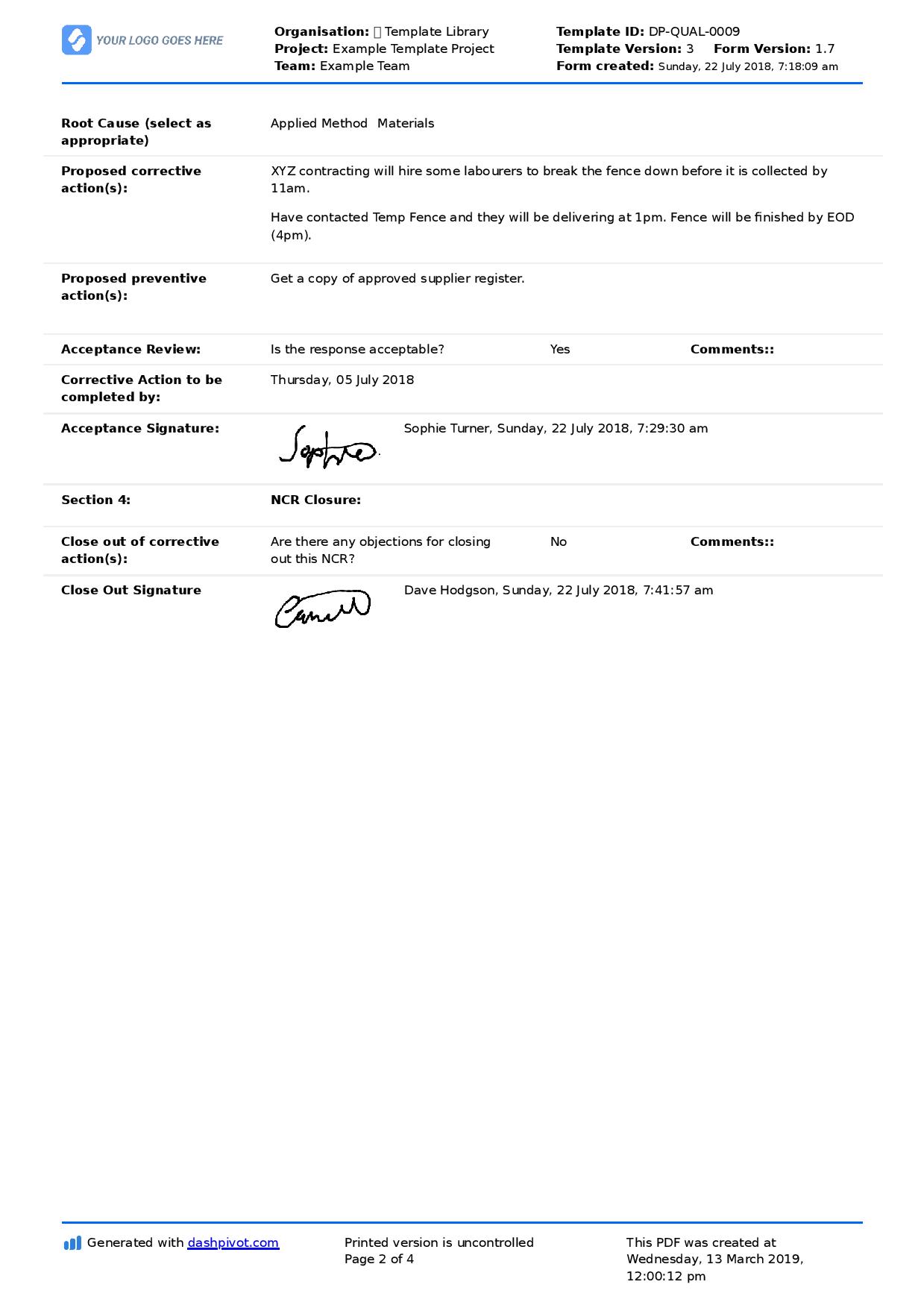
Corrective And Preventive Action Procedure - How to implement corrective and preventive action. Gmp sites should establish and. Corrective and preventive actions (capa) inspectional objectives. Food and drug administration (fda), corrective and preventive. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. This standard operating procedure describes the corrective and preventive action (capa) process.
This standard operating procedure describes the corrective and preventive action (capa) process. Corrective and preventive actions (capa) inspectional objectives. Gmp sites should establish and. How to implement corrective and preventive action. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Food and drug administration (fda), corrective and preventive.
Food and drug administration (fda), corrective and preventive. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. This standard operating procedure describes the corrective and preventive action (capa) process. How to implement corrective and preventive action. Corrective and preventive actions (capa) inspectional objectives. Gmp sites should establish and.
Corrective Action Plan Letter
Corrective and preventive actions (capa) inspectional objectives. This standard operating procedure describes the corrective and preventive action (capa) process. How to implement corrective and preventive action. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Food and drug administration (fda), corrective and preventive.
Procedure Corrective & Preventive Action.docx Business
Gmp sites should establish and. This standard operating procedure describes the corrective and preventive action (capa) process. How to implement corrective and preventive action. Food and drug administration (fda), corrective and preventive. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a.
Procedure for performing corrective/preventive action Download
Food and drug administration (fda), corrective and preventive. How to implement corrective and preventive action. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Corrective and preventive actions (capa) inspectional objectives. This standard operating procedure describes the corrective and preventive action (capa) process.
Corrective and preventive action procedure The right procedure for you
Gmp sites should establish and. Corrective and preventive actions (capa) inspectional objectives. How to implement corrective and preventive action. Food and drug administration (fda), corrective and preventive. This standard operating procedure describes the corrective and preventive action (capa) process.
Corrective and Preventive Action Procedure
This standard operating procedure describes the corrective and preventive action (capa) process. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Food and drug administration (fda), corrective and preventive. How to implement corrective and preventive action.
Corrective and Preventive Action Procedure
Gmp sites should establish and. Corrective and preventive actions (capa) inspectional objectives. How to implement corrective and preventive action. Food and drug administration (fda), corrective and preventive. This standard operating procedure describes the corrective and preventive action (capa) process.
Corrective and preventive action procedure The right procedure for you
Corrective and preventive actions (capa) inspectional objectives. How to implement corrective and preventive action. Food and drug administration (fda), corrective and preventive. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. This standard operating procedure describes the corrective and preventive action (capa) process.
Corrective Action and Preventive Action PDF Personal Protective
How to implement corrective and preventive action. Gmp sites should establish and. This standard operating procedure describes the corrective and preventive action (capa) process. Corrective and preventive actions (capa) inspectional objectives. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a.
Corrective and preventive action procedure The right procedure for you
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Gmp sites should establish and. How to implement corrective and preventive action. This standard operating procedure describes the corrective and preventive action (capa) process. Corrective and preventive actions (capa) inspectional objectives.
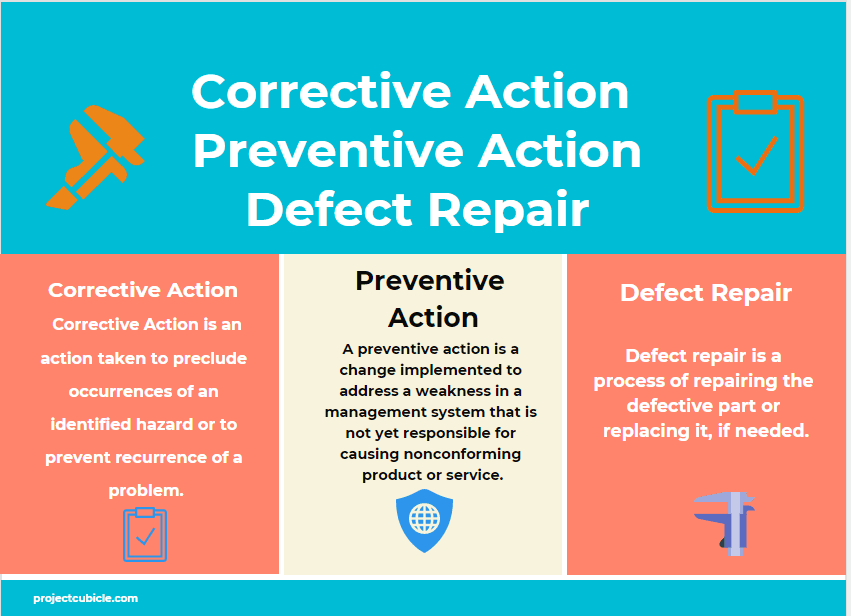
Corrective Action vs Preventive Action vs Defect Repair projectcubicle
This standard operating procedure describes the corrective and preventive action (capa) process. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. How to implement corrective and preventive action. Food and drug administration (fda), corrective and preventive. Gmp sites should establish and.
This Standard Operating Procedure Describes The Corrective And Preventive Action (Capa) Process.
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a. Gmp sites should establish and. Food and drug administration (fda), corrective and preventive. How to implement corrective and preventive action.