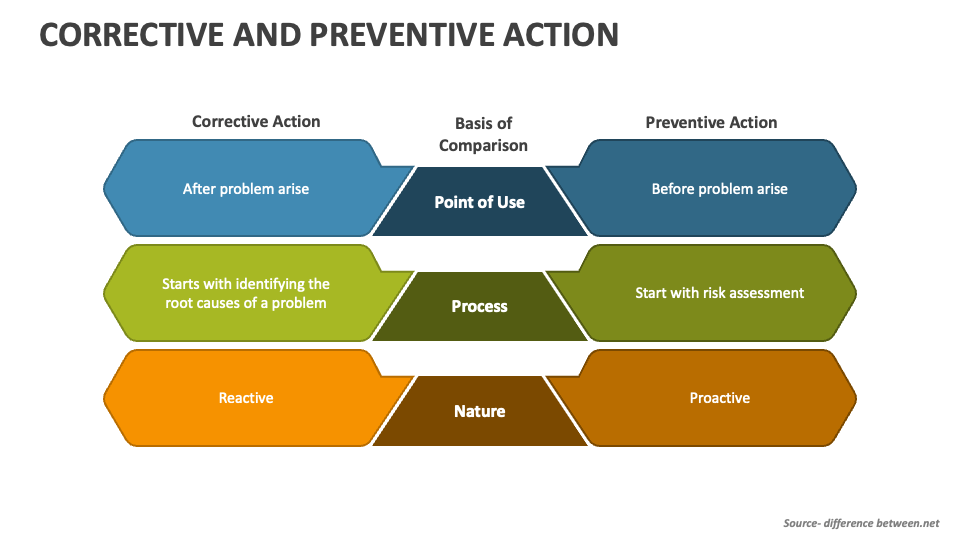
Corrective And Preventive Action - The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. Verify that capa system procedure (s) that address the requirements of the quality system regulation. Corrective and preventive actions (capa) inspectional objectives.
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Verify that capa system procedure (s) that address the requirements of the quality system regulation. Corrective and preventive actions (capa) inspectional objectives.
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Verify that capa system procedure (s) that address the requirements of the quality system regulation. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. Corrective and preventive actions (capa) inspectional objectives.
Corrective Action vs Preventive Action Difference and Comparison
The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. Verify that capa system procedure.
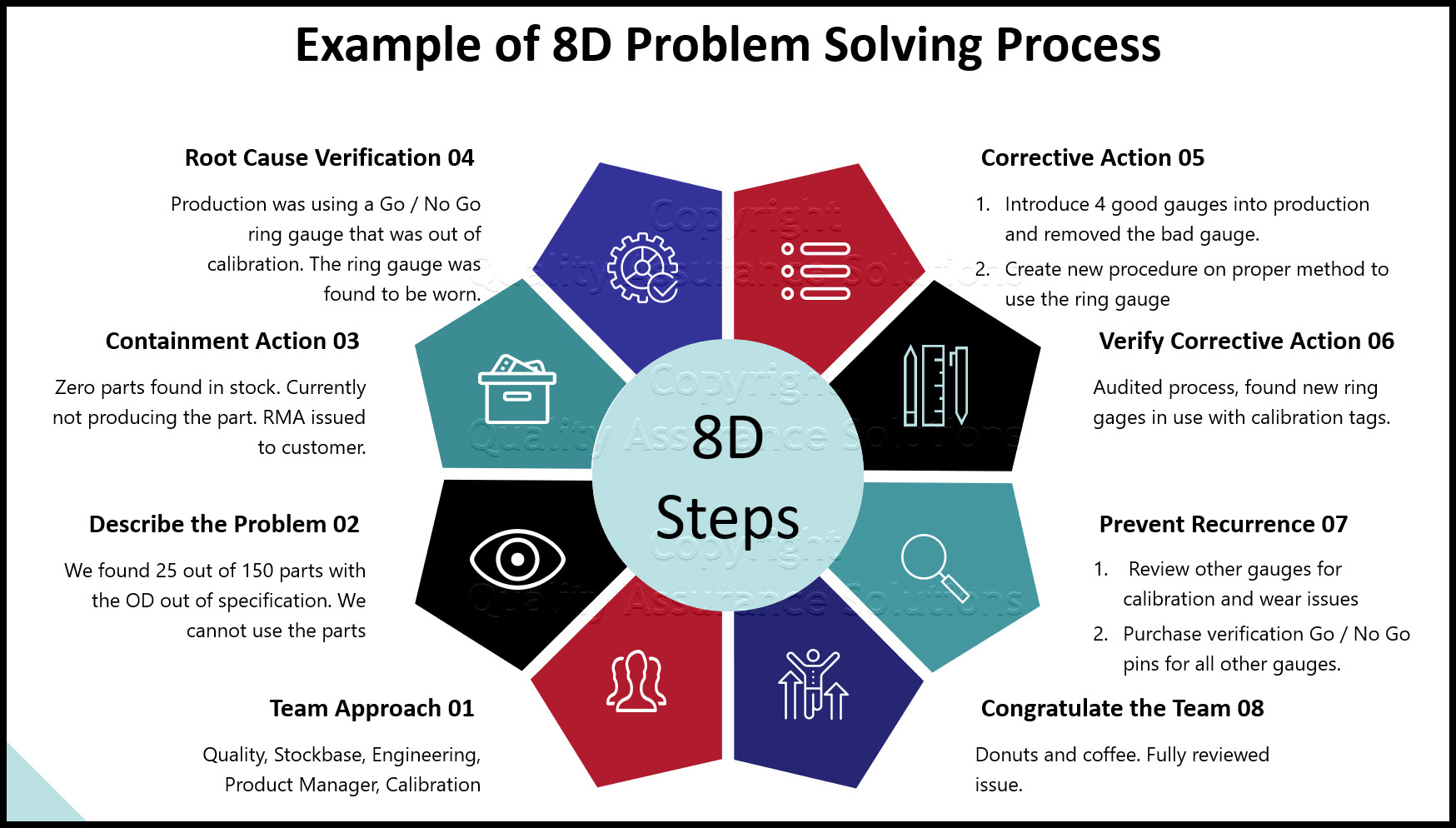
How to Use Corrective and Preventive Action (CAPA) To Deal With Non
Verify that capa system procedure (s) that address the requirements of the quality system regulation. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Corrective and preventive actions (capa) inspectional objectives. The purpose of corrective and preventive action is to collect and.
How to Use Corrective and Preventive Action (CAPA) To Deal With Non
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Verify that capa system procedure (s) that address the requirements of the quality system regulation. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and.
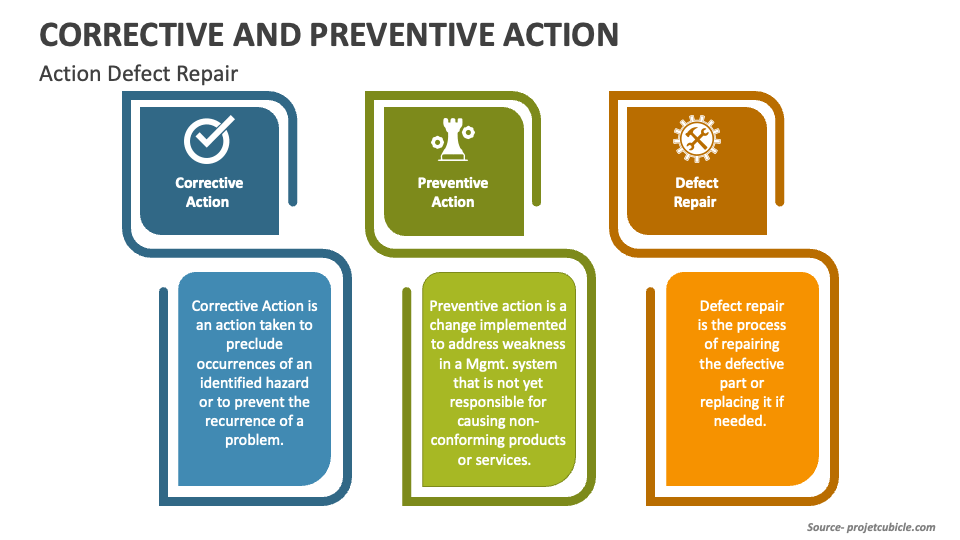
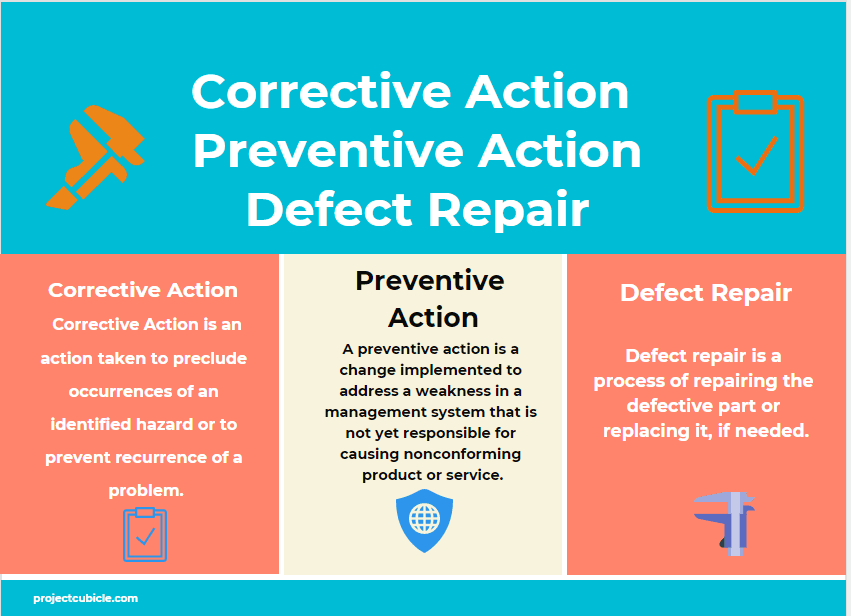
Perfect Your Project With Preventive, Corrective Action & Defect Repair
Corrective and preventive actions (capa) inspectional objectives. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take.
Corrective and Preventive Action Procedure
Verify that capa system procedure (s) that address the requirements of the quality system regulation. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Corrective and preventive actions (capa) inspectional objectives. Corrective actions (ca) take steps to fix the cause of a problem after the.
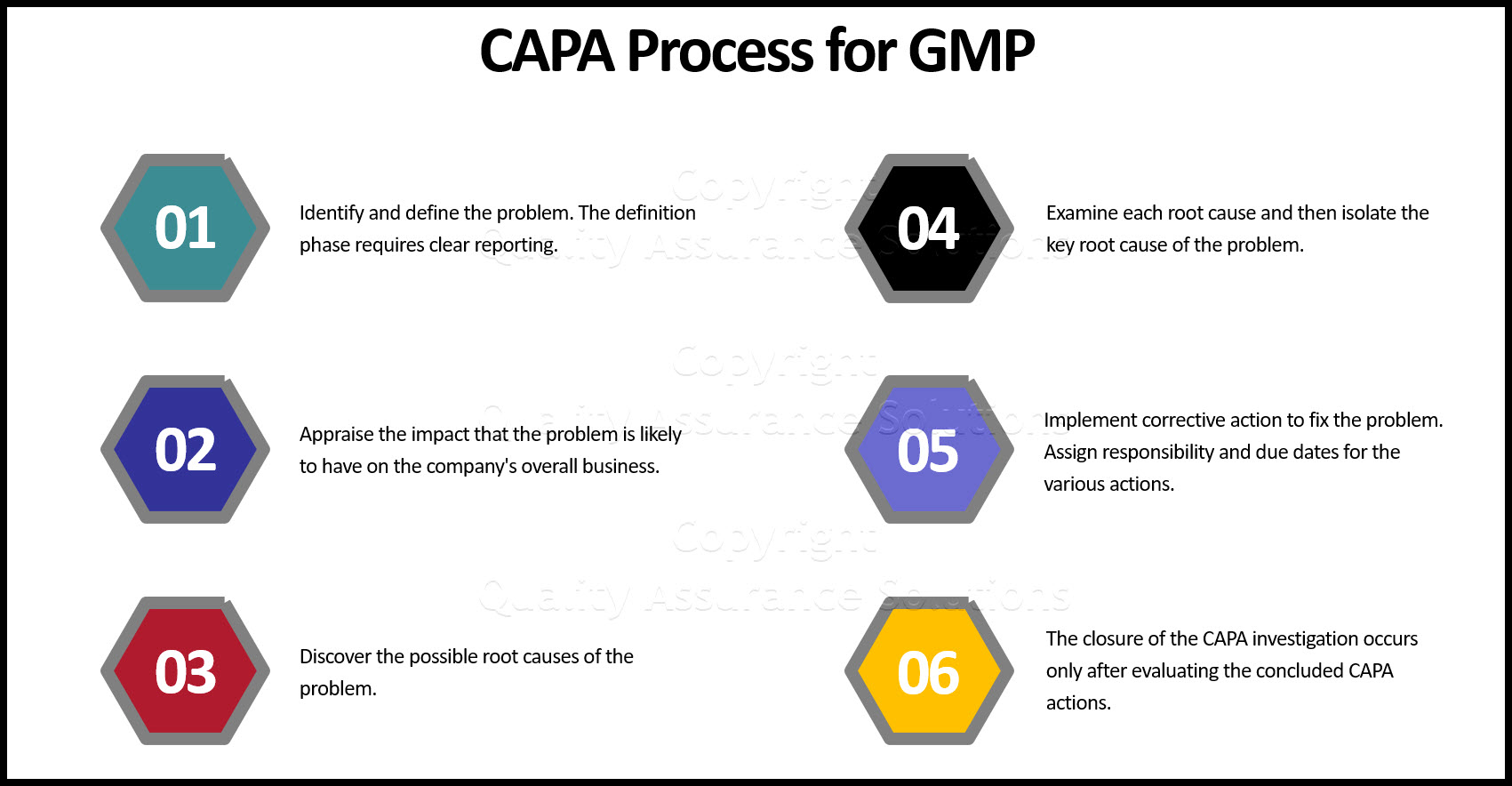
Preventive Corrective Action With 6 Steps
Verify that capa system procedure (s) that address the requirements of the quality system regulation. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Corrective and preventive actions (capa) inspectional objectives. Corrective actions (ca) take steps to fix the cause of a.
Corrective and Preventive Action PowerPoint Presentation Slides PPT
The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Verify that capa system procedure (s) that address the requirements of the quality system regulation. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and.
Preventive Corrective Action With 6 Steps
Verify that capa system procedure (s) that address the requirements of the quality system regulation. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa).
Corrective and Preventive Action PowerPoint Presentation Slides PPT
Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. Verify that capa system procedure.
Corrective Action vs Preventive Action vs Defect Repair projectcubicle
Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. Verify that capa system procedure (s) that address the requirements of the quality system regulation. Corrective and preventive actions (capa) inspectional objectives. The purpose of corrective and preventive action.
Corrective And Preventive Actions (Capa) Inspectional Objectives.
The purpose of corrective and preventive action is to collect and analyze information, identify and investigate product and quality problems, and take appropriate and. The corrective and preventive actions (capa) methodology is a beacon in this realm, providing a structured path to not only identifying and resolving nonconformities, but also preventing. Corrective actions (ca) take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions (pa) involve noticing the problem before it occurs, and taking steps. Verify that capa system procedure (s) that address the requirements of the quality system regulation.