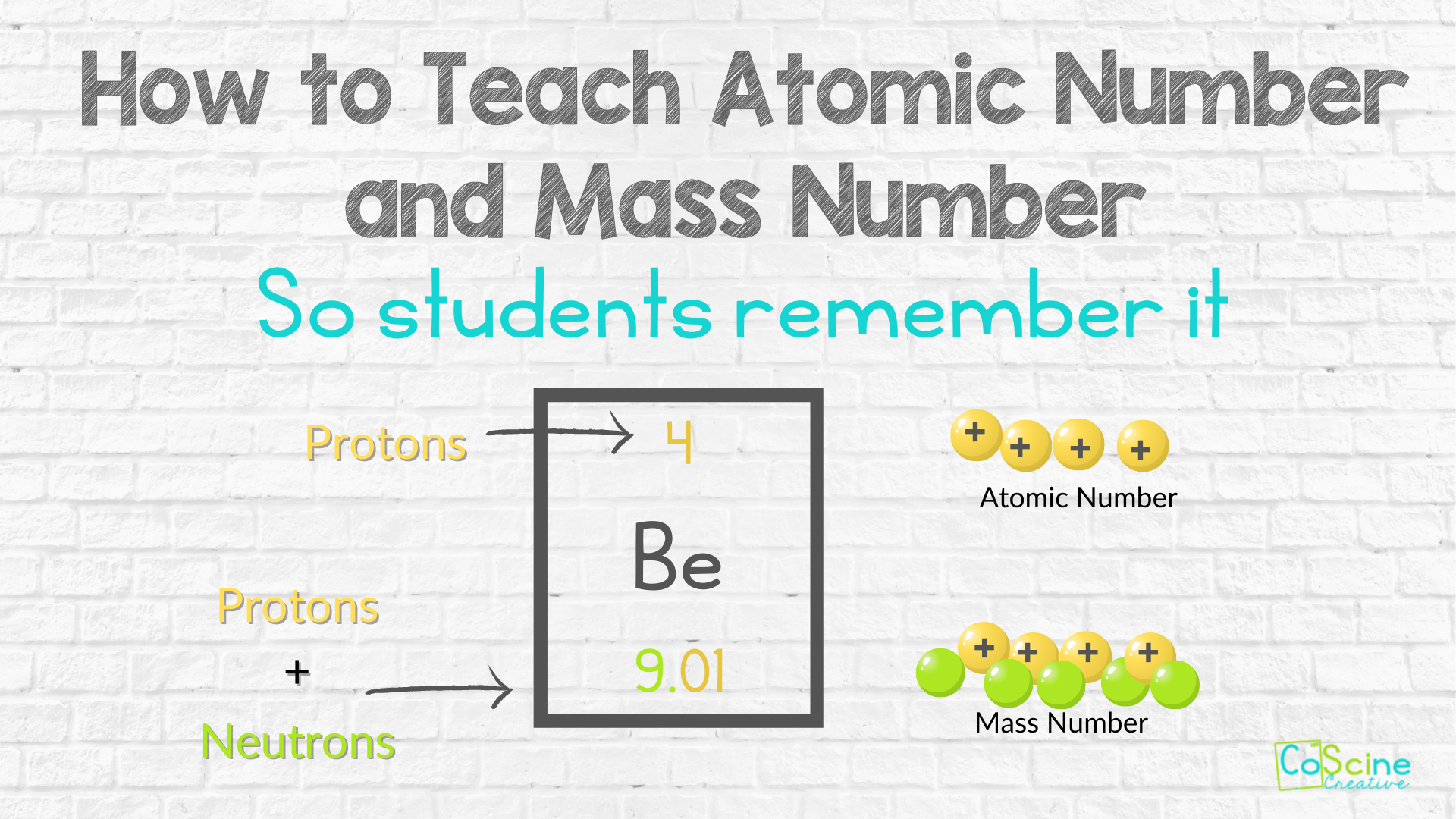
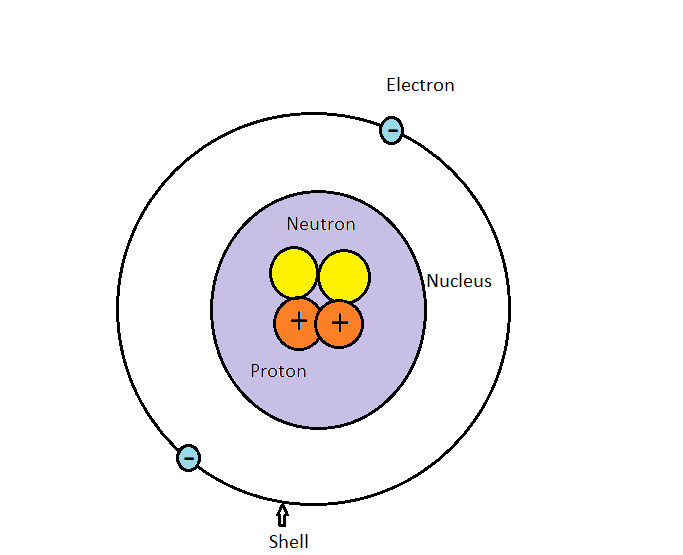
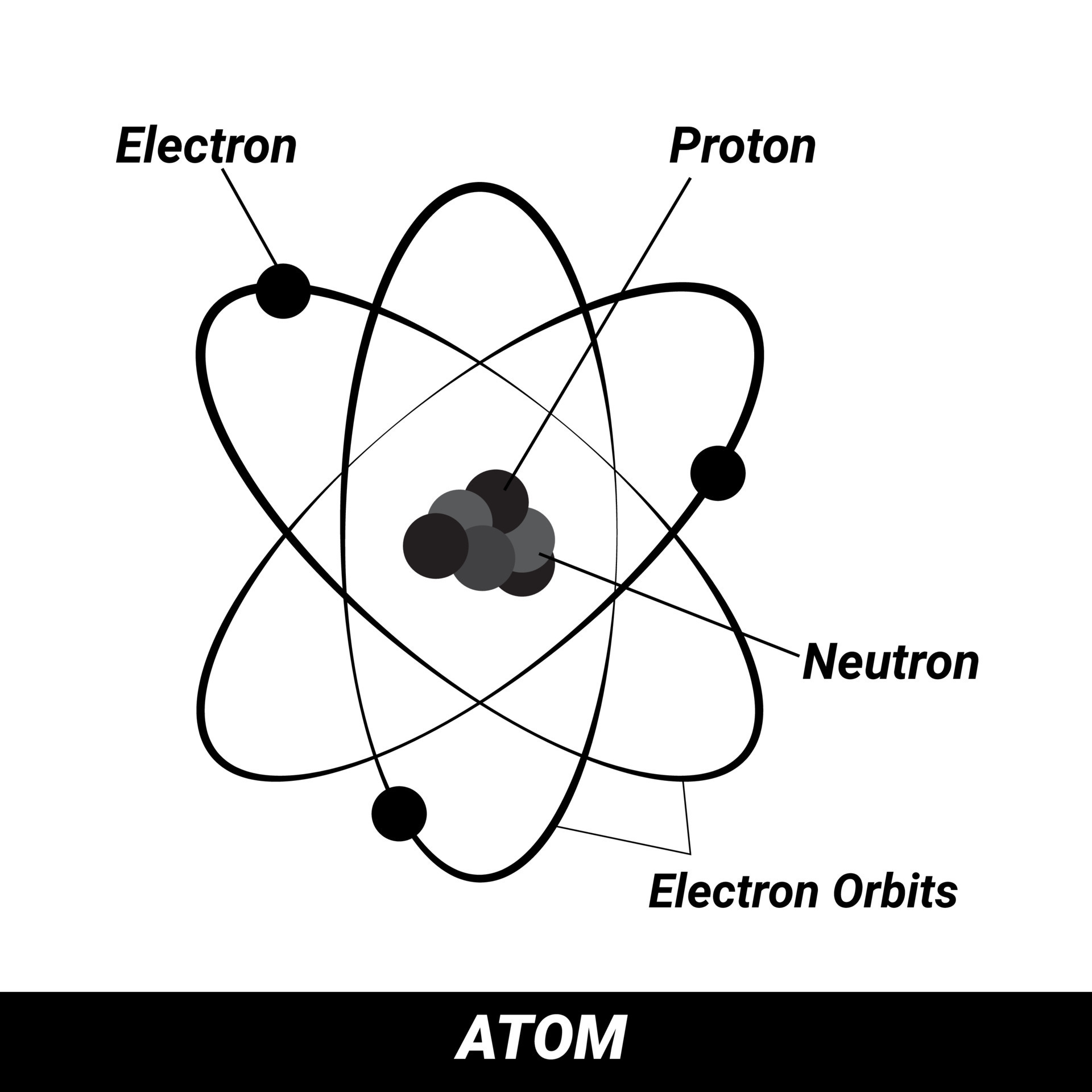
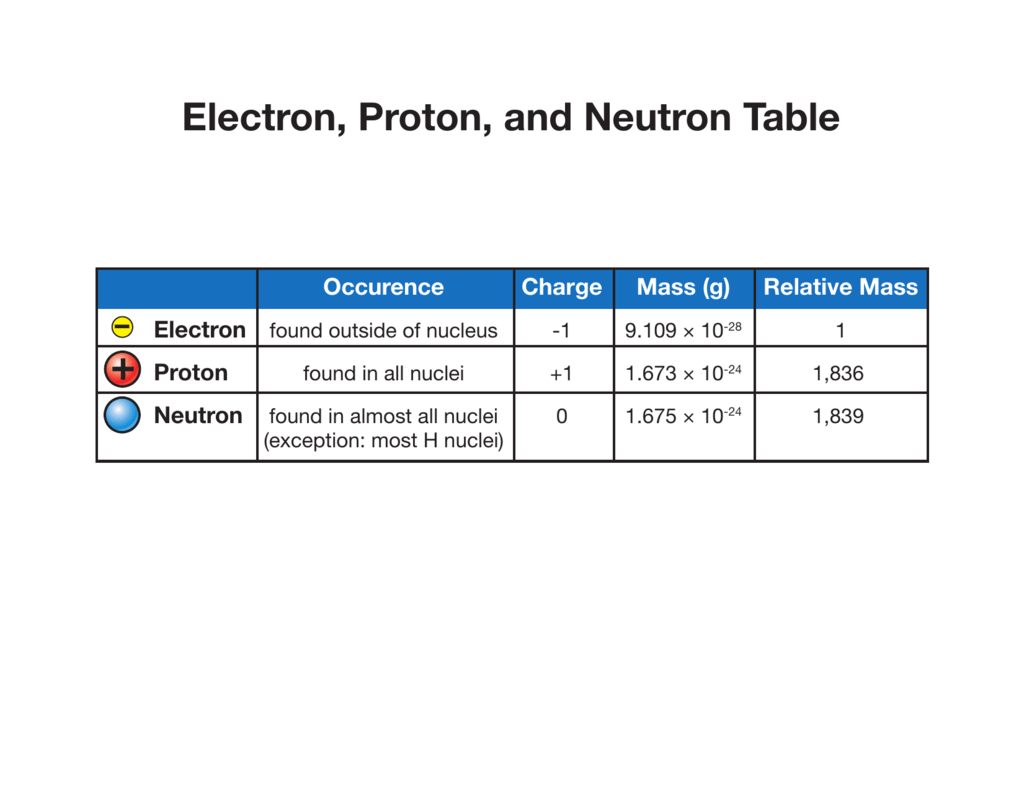
Differentiate Between Electron Proton And Neutron - Protons, electrons and neutrons have different properties like different charge, mass and stability. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Differentiate between electrons and protons. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons.
Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Protons, electrons and neutrons have different properties like different charge, mass and stability. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Differentiate between electrons and protons. Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons.
Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons, electrons and neutrons have different properties like different charge, mass and stability. Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Differentiate between electrons and protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and.
Proton Neutron Electron
Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Protons, electrons and neutrons have different.
Electron Proton Neutron
Protons, electrons and neutrons have different properties like different charge, mass and stability. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Find the relationship between one atom containing 12 protons,.
compare and contrast between electron,proton and neutron.
Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Differentiate between electrons and protons. Protons,.
Difference Between Proton, Neutron and Electrons
Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Protons, electrons and neutrons have different properties like different charge, mass and stability. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Protons and neutrons have approximately the same mass, but they are both much more massive than.
What are the Characteristics of Electron, Proton and Neutron A Plus
Protons, electrons and neutrons have different properties like different charge, mass and stability. Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. This article explains the differences between electrons, protons, and neutrons, including their charge,.
Electron Proton Neutron
Differentiate between electrons and protons. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Find.
What Is The Difference Between An Electron, Proton, And Neutron
Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons, electrons and neutrons have different properties like different charge, mass and stability. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Find the relationship between one atom containing 12 protons,.
Difference Between Proton, Neutron and Electrons
Protons, electrons and neutrons have different properties like different charge, mass and stability. Differentiate between electrons and protons. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Find the relationship between.
Proton Electron Neutron Venn Diagram Neutron Atom Aluminium
Differentiate between electrons and protons. This article explains the differences between electrons, protons, and neutrons, including their charge, location, mass, and. Find the relationship between one atom containing 12 protons, 12 electrons, and 12 neutrons and. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Protons, electrons and neutrons have.
Electron, Proton, and Neutron Table
Protons, electrons and neutrons have different properties like different charge, mass and stability. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. This article explains the differences between electrons, protons, and.
This Article Explains The Differences Between Electrons, Protons, And Neutrons, Including Their Charge, Location, Mass, And.
Protons, electrons and neutrons have different properties like different charge, mass and stability. Electrons are negatively charged subatomic particles found in the electron cloud around the nucleus of an atom, whereas protons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Differentiate between electrons and protons.