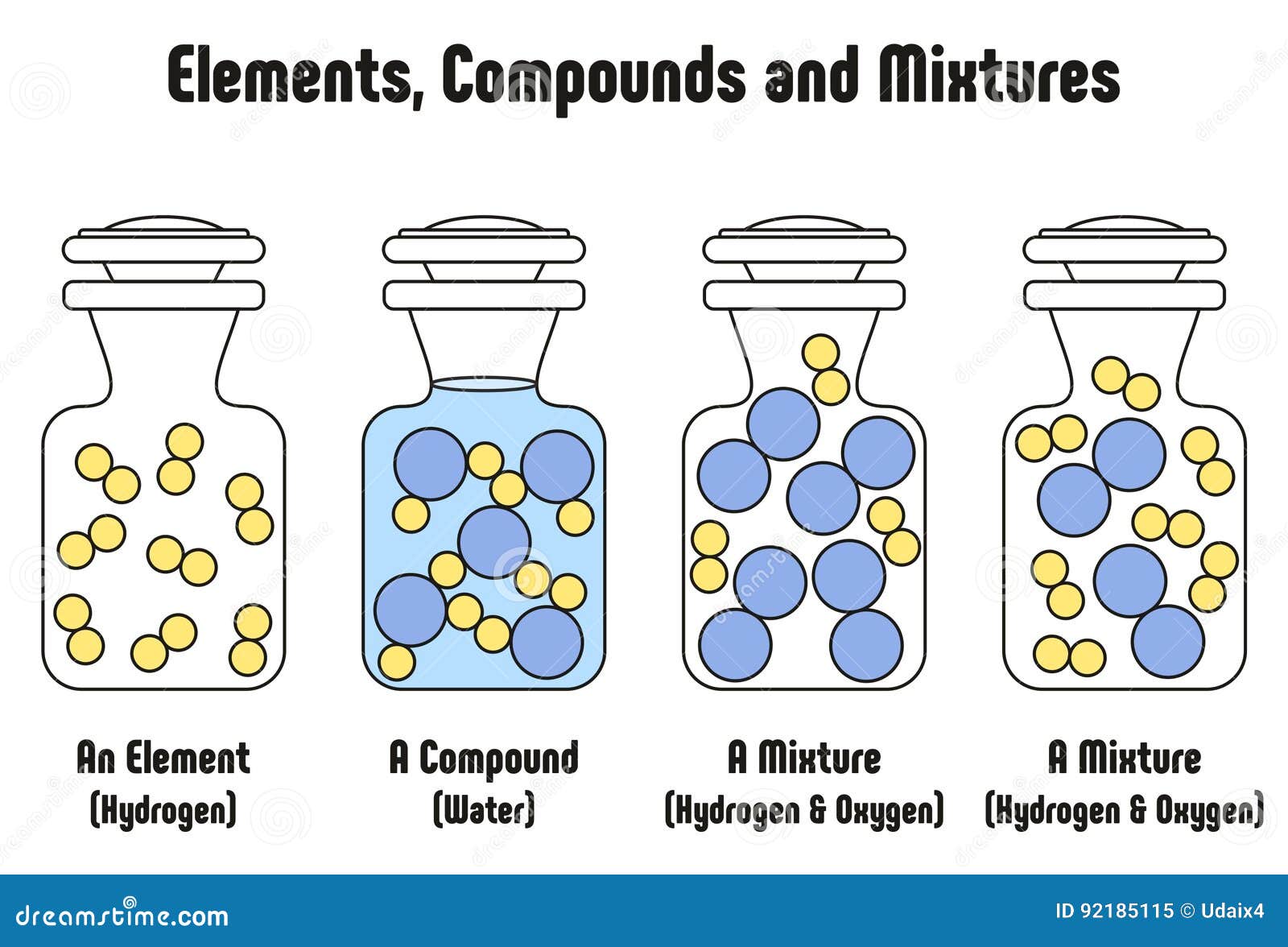
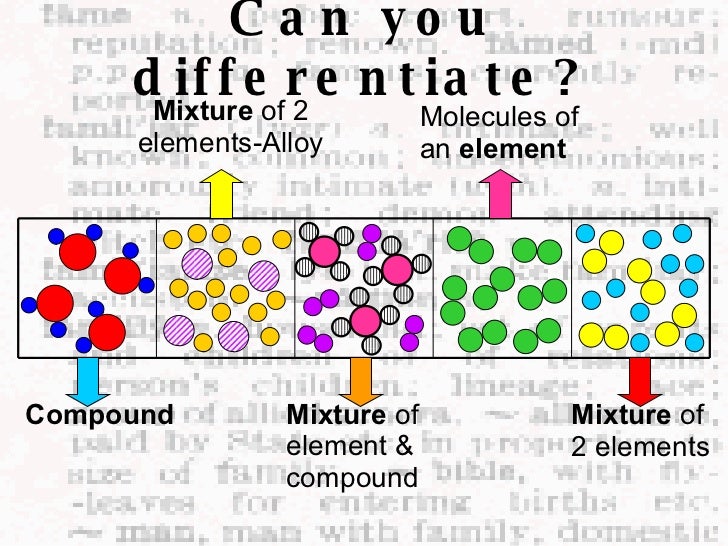
Differentiate Between Elements Compounds And Mixtures - The elements in a chemical compound can only be separated by destroying the compound. The main difference between an element, a compound, and a mixture is their composition and chemical properties. They do not contain any. Chemical compounds are very different from mixtures: Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded.
The elements in a chemical compound can only be separated by destroying the compound. Chemical compounds are very different from mixtures: Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. The main difference between an element, a compound, and a mixture is their composition and chemical properties. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; They do not contain any.
The elements in a chemical compound can only be separated by destroying the compound. Chemical compounds are very different from mixtures: Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. The main difference between an element, a compound, and a mixture is their composition and chemical properties. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; They do not contain any.
Compounds Vs Elements Vs Mixtures Foto Kolekcija
They do not contain any. The elements in a chemical compound can only be separated by destroying the compound. The main difference between an element, a compound, and a mixture is their composition and chemical properties. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Chemical compounds are very.
Elements Compounds or Mixtures worksheet Compounds and mixtures
Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Chemical compounds are very different from mixtures: Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. They do not contain any. The main difference between an element, a compound, and a mixture is.
Elements Compounds And Mixtures Test Quizlet EduForKid
The elements in a chemical compound can only be separated by destroying the compound. Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; The main difference between an element, a compound, and a.
Elements, Compounds, Mixtures
The main difference between an element, a compound, and a mixture is their composition and chemical properties. They do not contain any. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Chemical compounds are very different from mixtures: Mixtures are combinations of two or more substances (elements or compounds).
Elements Compounds And Mixtures Differences Foto Kolekcija
Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the compound. Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. They do not contain any. The main difference between an element, a compound, and a mixture is their composition and chemical properties.
What's the difference between Elements, Compounds and Mixtures? In My
They do not contain any. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; The elements in a chemical compound can only be separated by destroying the compound. The main difference between an element, a compound, and a mixture is their composition and chemical properties. Mixtures are combinations of.
Differences between Elements, Compounds & Mixtures Compounds and
Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; The main difference between an element, a compound, and a mixture is their composition and chemical properties. The elements in a chemical compound can only be separated by destroying the compound. They do not contain any. Chemical compounds are very.
Elements Compounds And Mixtures Worksheet Grade 5 1088038 Free
The main difference between an element, a compound, and a mixture is their composition and chemical properties. Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the compound. They do not contain any.
Elements, Compounds, Mixtures
They do not contain any. The elements in a chemical compound can only be separated by destroying the compound. Mixtures are combinations of two or more substances (elements or compounds) that are not chemically bonded. The main difference between an element, a compound, and a mixture is their composition and chemical properties. Chemical compounds are very different from mixtures:
Elements, Compounds, and Mixtures Diagram Quizlet
Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Chemical compounds are very different from mixtures: They do not contain any. The elements in a chemical compound can only be separated by destroying the compound. Mixtures are combinations of two or more substances (elements or compounds) that are not.
The Main Difference Between An Element, A Compound, And A Mixture Is Their Composition And Chemical Properties.
Chemical compounds are very different from mixtures: Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; The elements in a chemical compound can only be separated by destroying the compound. They do not contain any.