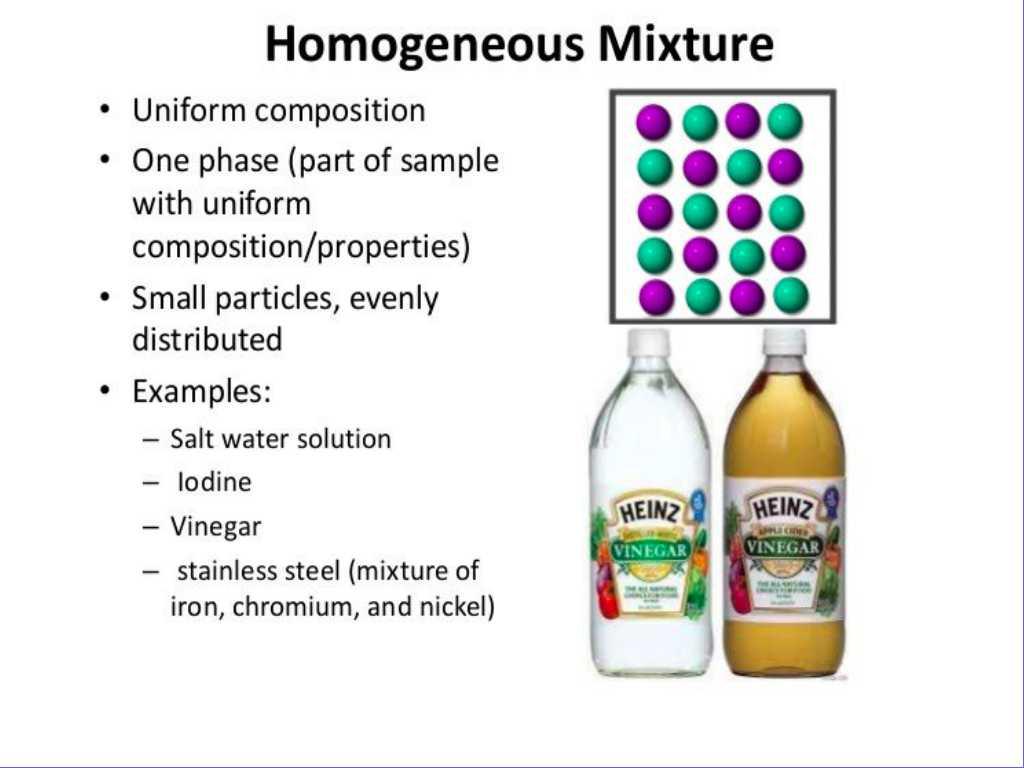
Differentiate Between Heterogeneous And Homogeneous Mixtures - Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous? Mixtures are different from pure substances like elements and compounds.
Mixtures are different from pure substances like elements and compounds. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous?
Mixtures are different from pure substances like elements and compounds. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous?
Difference Between Heterogeneous And Homogeneous Mixtures, 46 OFF
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. Mixtures are different from pure substances like elements and compounds. What's the difference between heterogeneous and homogeneous?
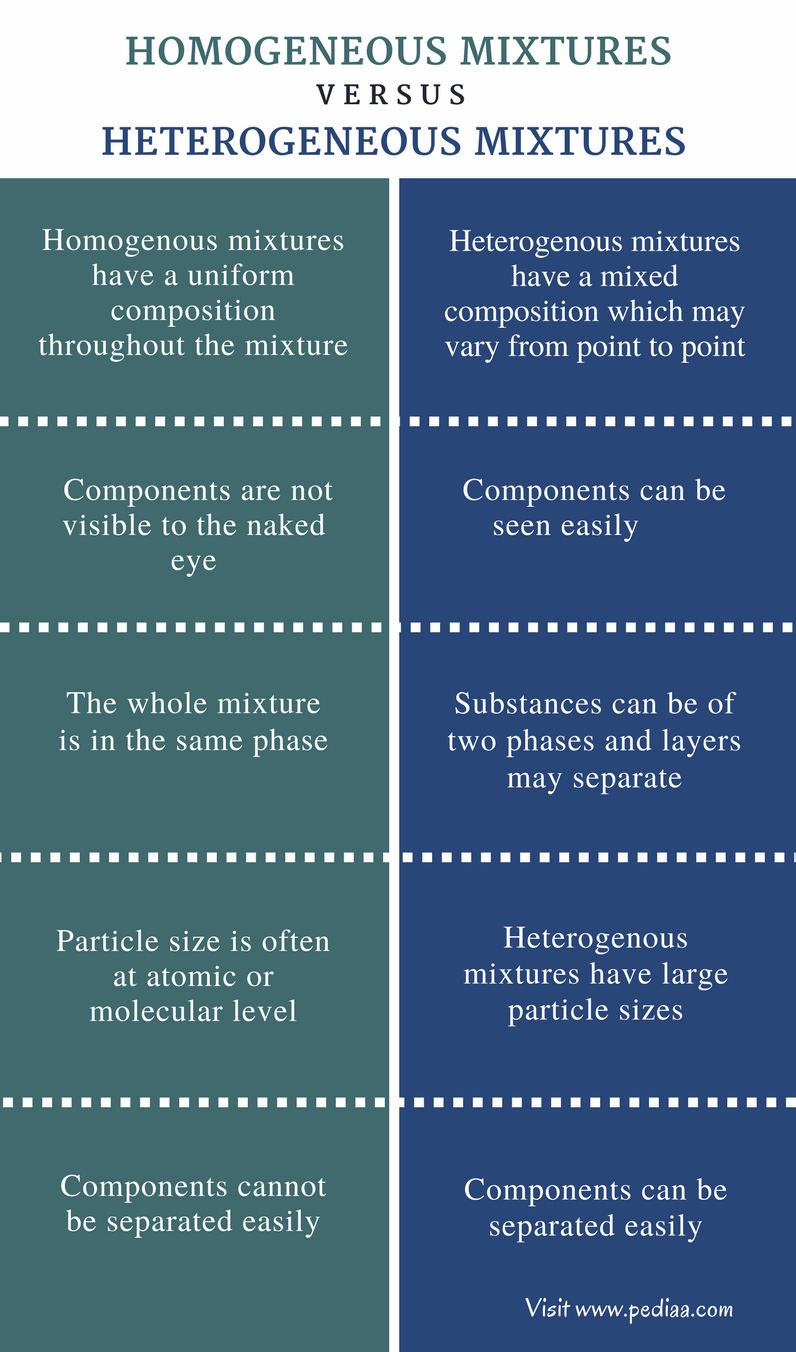
Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous? Mixtures are different from pure substances like elements and compounds.
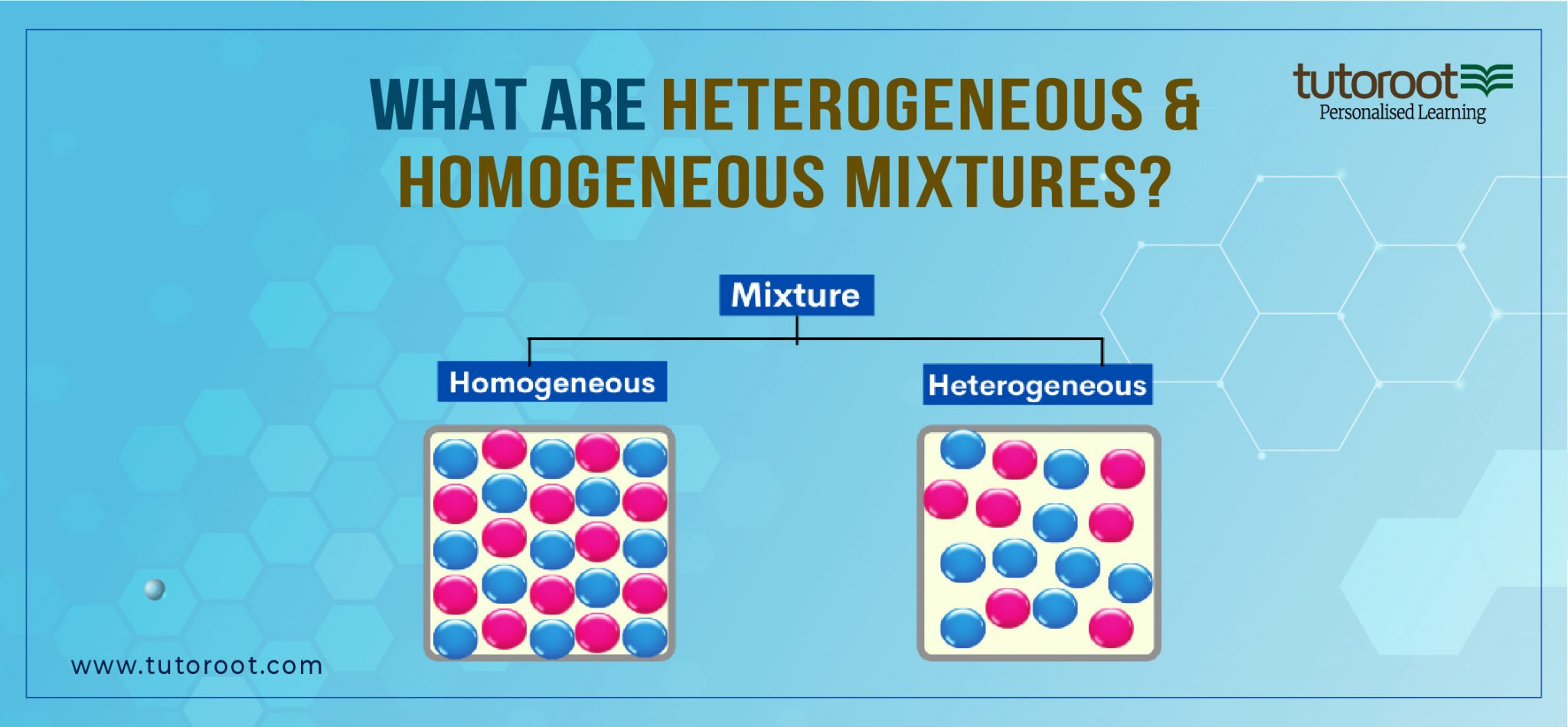
What are Heterogeneous and Homogeneous Mixtures? Tutoroot
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous? Mixtures are different from pure substances like elements and compounds.
Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous? Mixtures are different from pure substances like elements and compounds.
Differentiate between homogeneous and heterogeneous mixtures by giving a..
Mixtures are different from pure substances like elements and compounds. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous?
Difference Between Homogeneous and Heterogeneous Mixtures Definition
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous? Mixtures are different from pure substances like elements and compounds.
Differentiate between Homogeneous and Heterogeneous mixture with
Mixtures are different from pure substances like elements and compounds. What's the difference between heterogeneous and homogeneous? Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been.
Heterogeneous vs. Homogeneous Mixtures
What's the difference between heterogeneous and homogeneous? Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. Mixtures are different from pure substances like elements and compounds.
CHEMISTRY MIXTURES HOMOGENEOUS & HETEROGENEOUS
Mixtures are different from pure substances like elements and compounds. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. What's the difference between heterogeneous and homogeneous?
1. Differentiate between homogeneous and heterogeneous mixtures with exam..
What's the difference between heterogeneous and homogeneous? Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been. Mixtures are different from pure substances like elements and compounds.
Scientifically Speaking, A Homogeneous Mixture Is One In Which Different Parts (Such As Salt And Water) Have Been.
Mixtures are different from pure substances like elements and compounds. What's the difference between heterogeneous and homogeneous?




/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)