Does Positive Pressure Prevent Volatile Compounds From Leabing - The behaviors of ideal solutions of volatile compounds follow raoult’s law. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the. In chemistry and physics, volatility is the tendency of a substance to vaporize. Henry’s law can be used to describe the deviations. Volatility is directly related to a substance's vapor.
In chemistry and physics, volatility is the tendency of a substance to vaporize. The behaviors of ideal solutions of volatile compounds follow raoult’s law. Henry’s law can be used to describe the deviations. Volatility is directly related to a substance's vapor. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the.
In chemistry and physics, volatility is the tendency of a substance to vaporize. The behaviors of ideal solutions of volatile compounds follow raoult’s law. Volatility is directly related to a substance's vapor. Henry’s law can be used to describe the deviations. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point.
Volatile Organic Compounds Eurofins Scientific
Volatility is directly related to a substance's vapor. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the. Henry’s law can be used to describe the deviations. The behaviors of ideal solutions of volatile compounds follow raoult’s law. (by the way, we know that pressure is.
Volatile organic compounds used in this work. Download Scientific Diagram
Henry’s law can be used to describe the deviations. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. In chemistry and physics, volatility is the tendency of a substance to vaporize. The behaviors of ideal solutions of volatile compounds follow raoult’s law. If the.
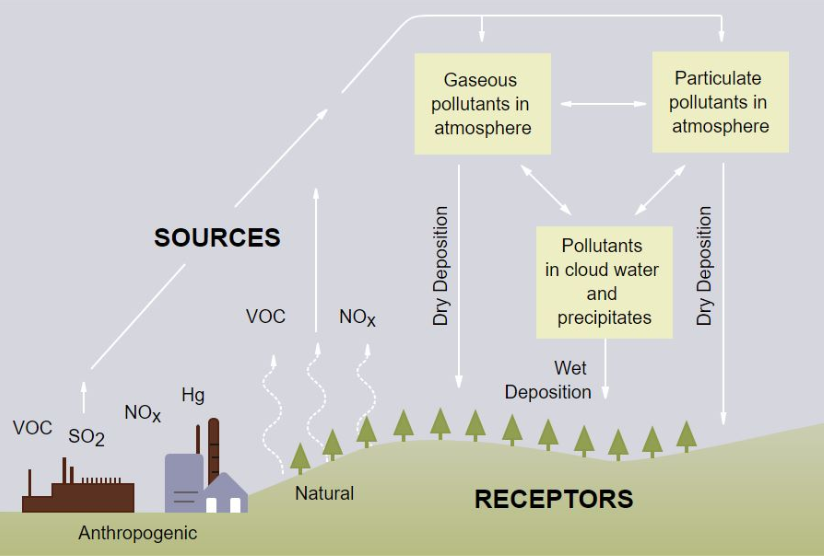
How does volatile organic compounds concentration affect the environment
Henry’s law can be used to describe the deviations. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. Volatility is directly related to a substance's vapor. In chemistry and physics, volatility is the tendency of a substance to vaporize. The behaviors of ideal solutions.
Demystifying the study of volatile organic plant compounds
(by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. In chemistry and physics, volatility is the tendency of a substance to vaporize. The behaviors of ideal solutions of volatile compounds follow raoult’s law. Henry’s law can be used to describe the deviations. Volatility is.
Low Vapor PressureVolatile Organic Compounds at Florencio Sam blog
(by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. Volatility is directly related to a substance's vapor. Henry’s law can be used to describe the deviations. The behaviors of ideal solutions of volatile compounds follow raoult’s law. In chemistry and physics, volatility is the.
Beaker with volatile compounds evaporating under low pressure, chemical
Henry’s law can be used to describe the deviations. Volatility is directly related to a substance's vapor. The behaviors of ideal solutions of volatile compounds follow raoult’s law. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the. In chemistry and physics, volatility is the tendency.
What are the LongTerm Effects of Volatile Organic Compounds?
Volatility is directly related to a substance's vapor. In chemistry and physics, volatility is the tendency of a substance to vaporize. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. The behaviors of ideal solutions of volatile compounds follow raoult’s law. Henry’s law can.
Low Vapor PressureVolatile Organic Compounds at Florencio Sam blog
In chemistry and physics, volatility is the tendency of a substance to vaporize. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. Henry’s law can be used to describe the deviations. Volatility is directly related to a substance's vapor. The behaviors of ideal solutions.
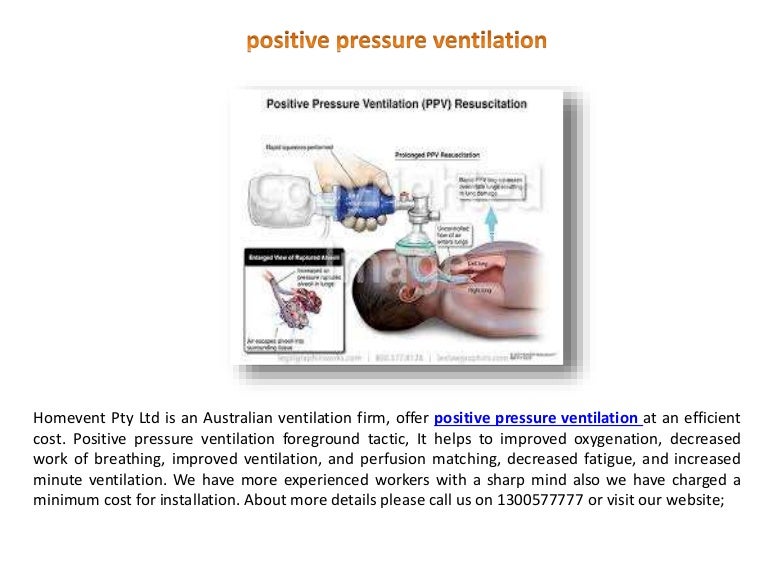
Positive pressure ventilation
The behaviors of ideal solutions of volatile compounds follow raoult’s law. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the..
How does volatile organic compounds concentration affect the environment
Henry’s law can be used to describe the deviations. (by the way, we know that pressure is directly proportional to the number of moles from consideration of the ideal gas law.) a key point. Volatility is directly related to a substance's vapor. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each.
(By The Way, We Know That Pressure Is Directly Proportional To The Number Of Moles From Consideration Of The Ideal Gas Law.) A Key Point.
In chemistry and physics, volatility is the tendency of a substance to vaporize. If the liquid inside a container is a mixture of several volatile compounds, then the vapors of each compound will exert pressure on the. Henry’s law can be used to describe the deviations. Volatility is directly related to a substance's vapor.



.jpg)