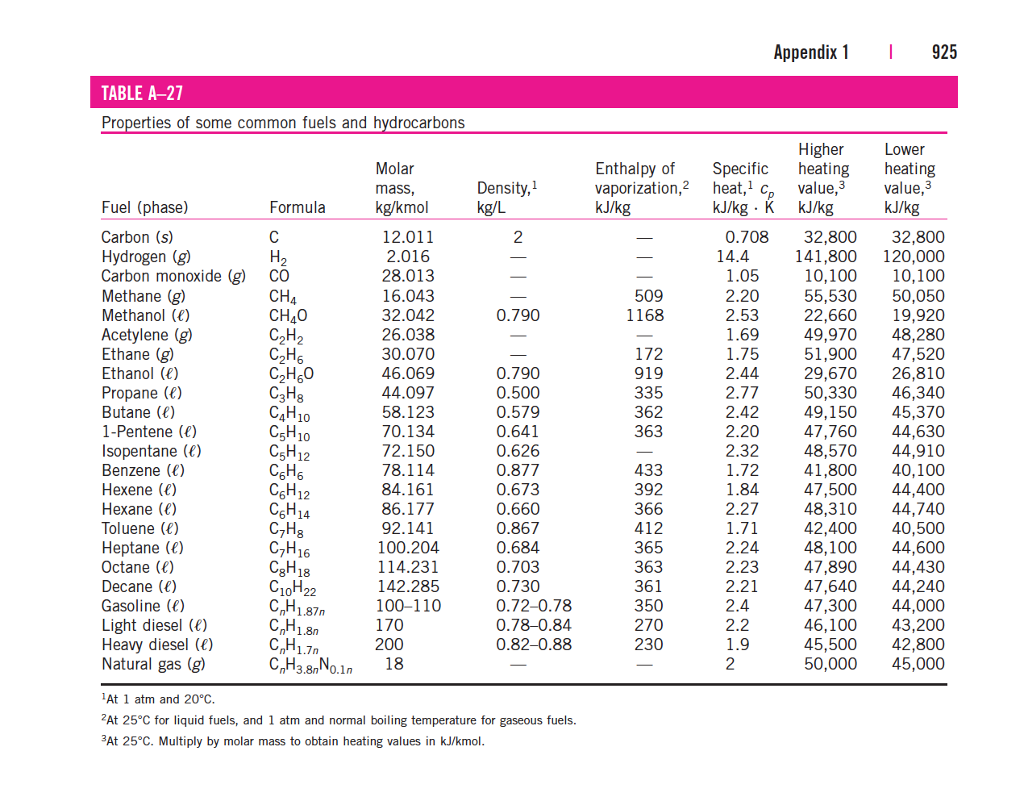
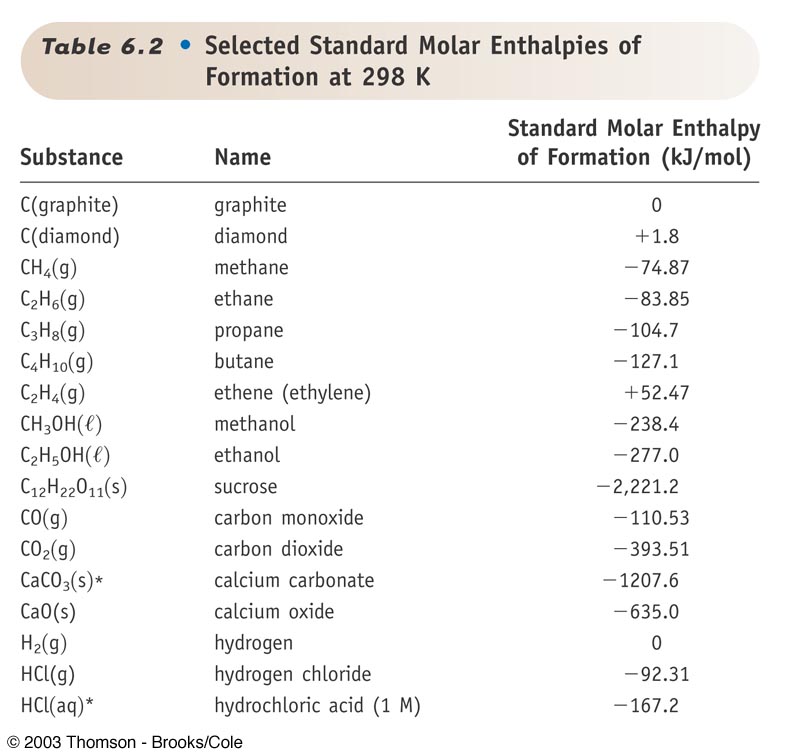
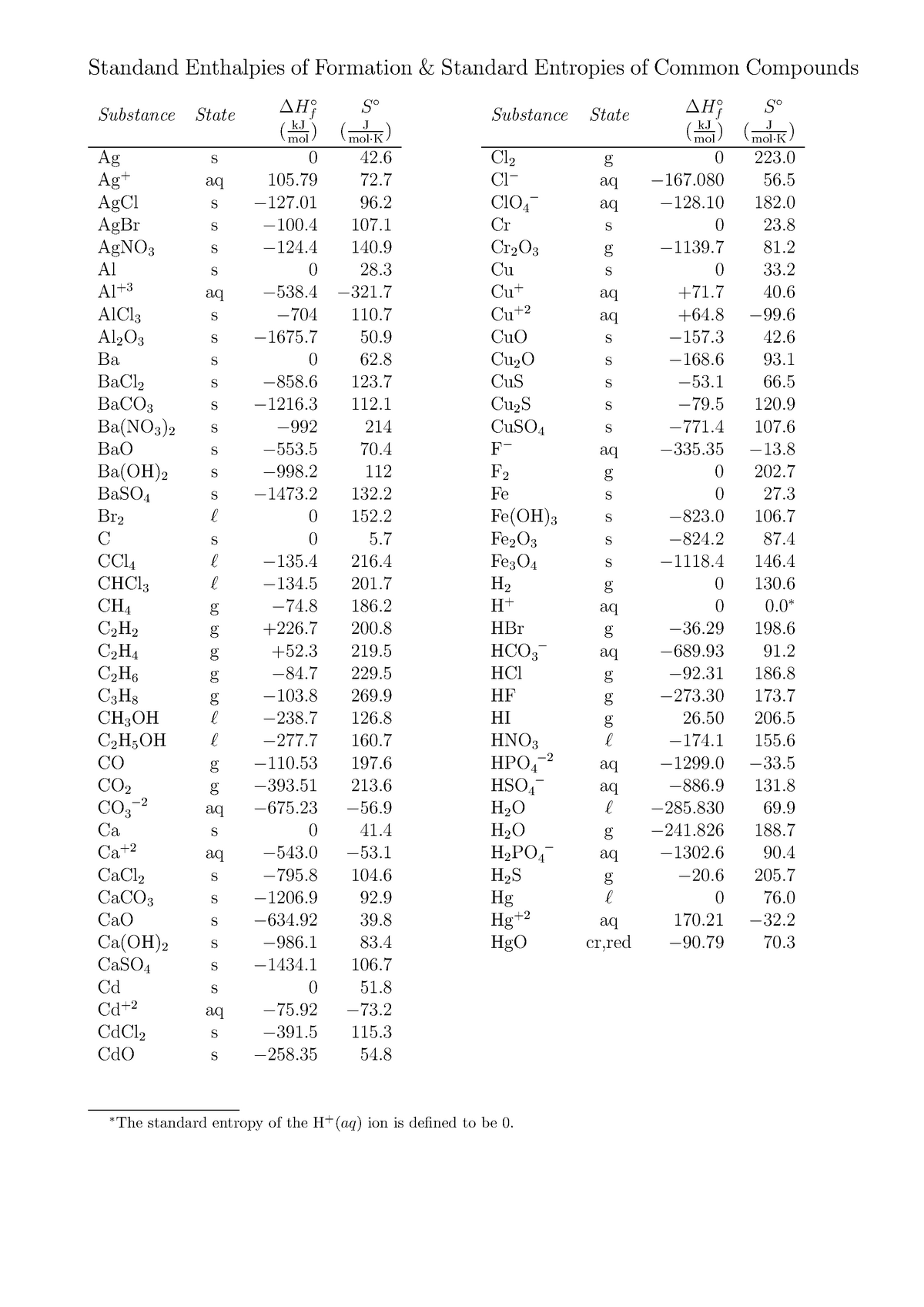
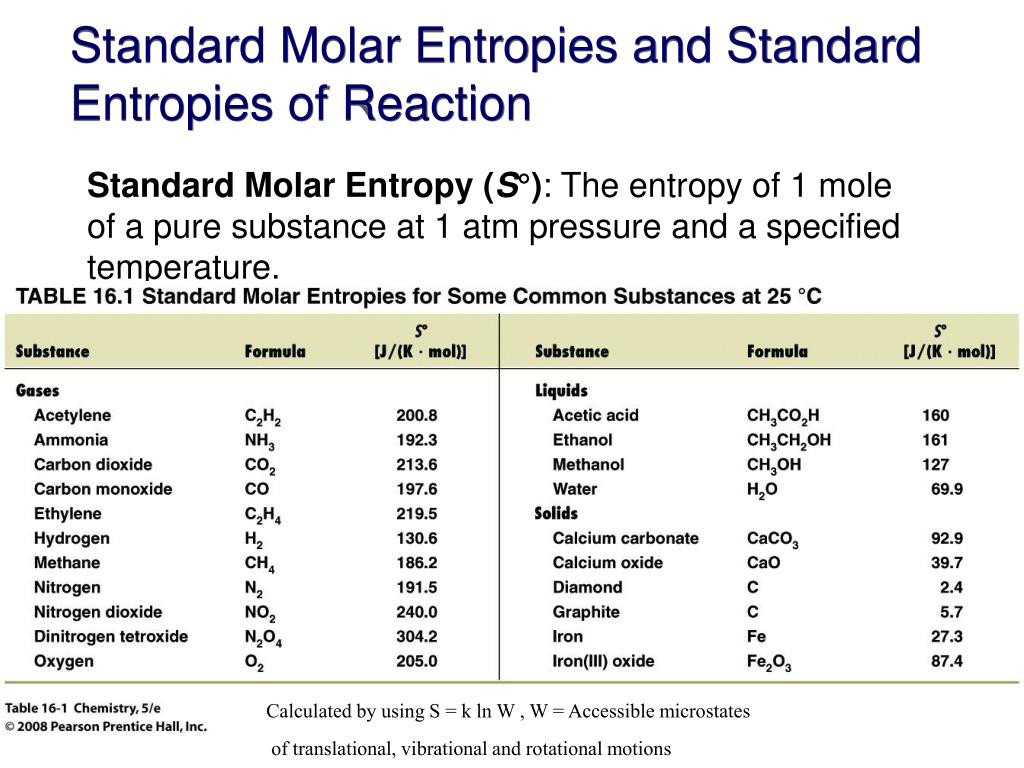
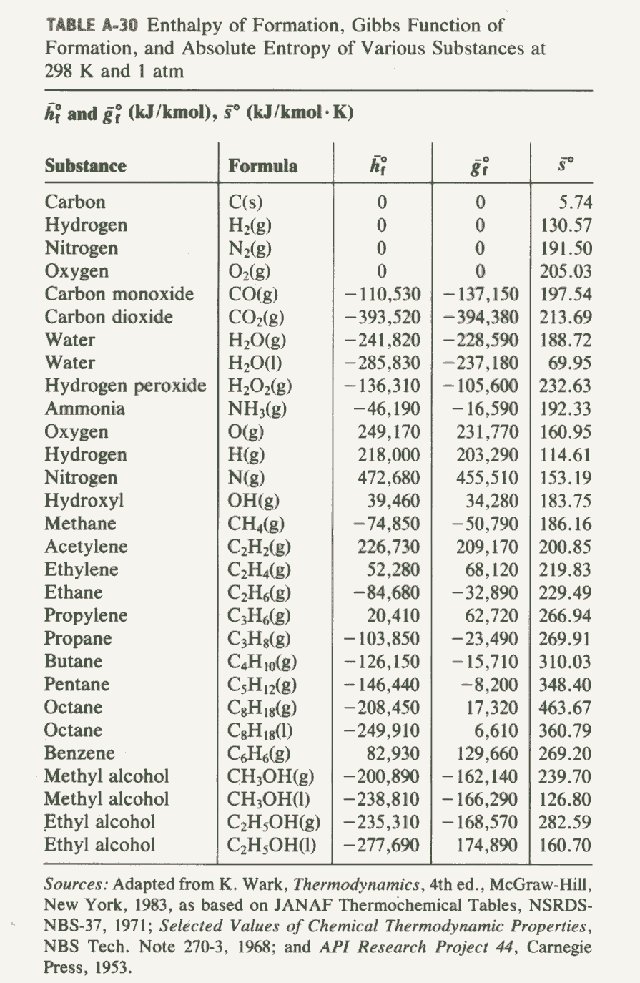
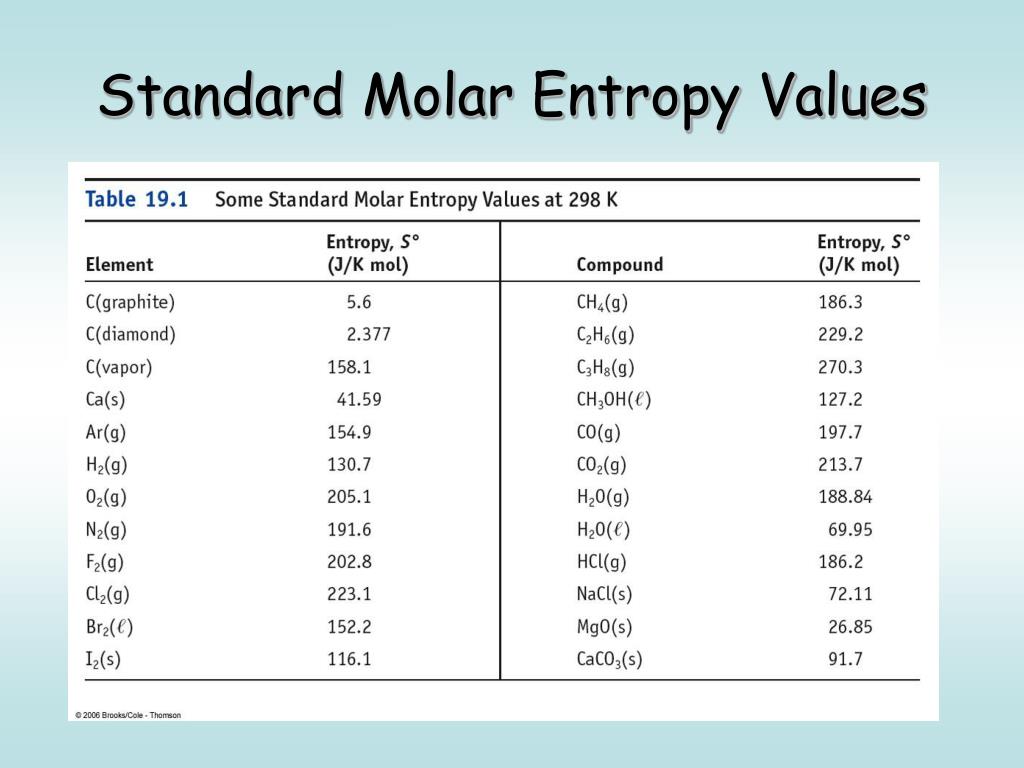
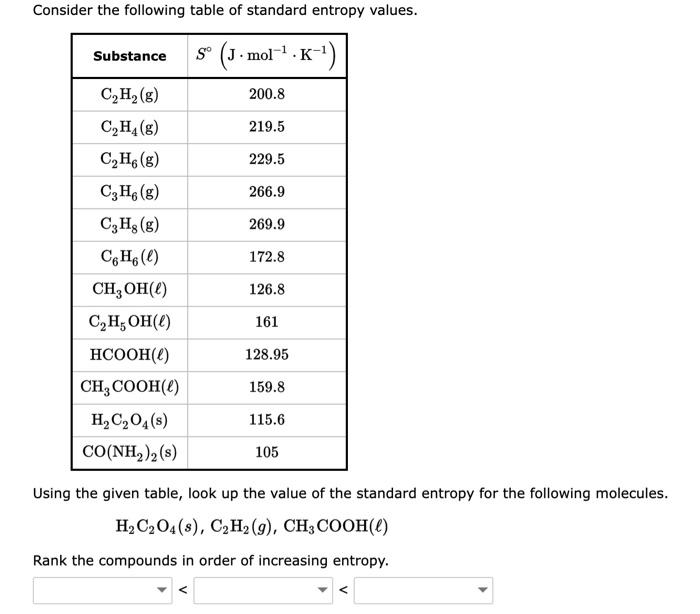
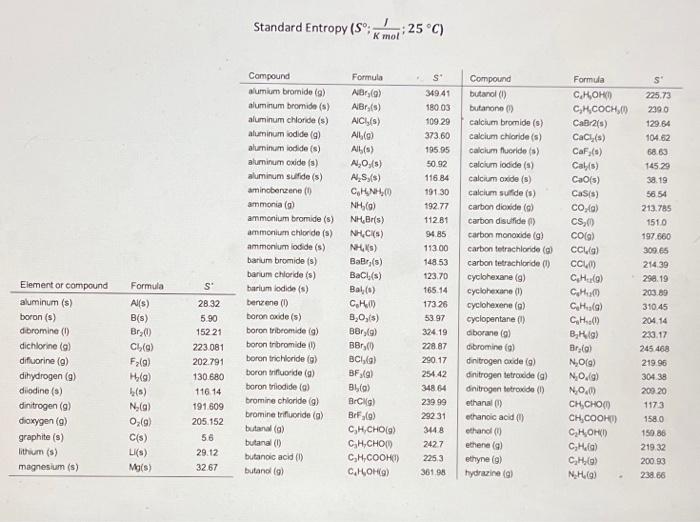
Standard Entropy Of Formation Table - 100 rows standard heats and free energies of formation and absolute entropies of elements. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. ∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Values of the standard molar entropies of various substances at 298 k (25°c). 151 rows internationally agreed, internally consistent, values for the thermodynamic. Definition and explanation of the terms standard state and standard enthalpy of formation, with.
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. 100 rows standard heats and free energies of formation and absolute entropies of elements. Values of the standard molar entropies of various substances at 298 k (25°c). 151 rows internationally agreed, internally consistent, values for the thermodynamic. Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0.
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Values of the standard molar entropies of various substances at 298 k (25°c). Definition and explanation of the terms standard state and standard enthalpy of formation, with. 151 rows internationally agreed, internally consistent, values for the thermodynamic. ∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. 100 rows standard heats and free energies of formation and absolute entropies of elements.
TABLE A286 Enthalpy of formation, Gibbs function of
The table below shows the standard enthalpy of formation, the standard gibbs free energy of. ∗the standard entropy of the h+(aq) ion is defined to be 0. 151 rows internationally agreed, internally consistent, values for the thermodynamic. Definition and explanation of the terms standard state and standard enthalpy of formation, with. This table lists the standard enthalpies (δh°), the free.
[Solved] Calculate the standard molar entropy change for the formation
151 rows internationally agreed, internally consistent, values for the thermodynamic. ∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. Definition and explanation of the terms standard state and standard enthalpy of formation, with. This table lists the standard enthalpies (δh°), the free.
to Adobe GoLive 6
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. ∗the standard entropy of the h+(aq) ion is defined to be 0. 151 rows internationally agreed, internally consistent, values for the thermodynamic. Values of the standard molar entropies of various substances at 298 k (25°c). 100 rows standard heats and free energies of formation and.
Standard entropy table Lasialabama
Values of the standard molar entropies of various substances at 298 k (25°c). 100 rows standard heats and free energies of formation and absolute entropies of elements. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. Definition and explanation of the terms standard state and standard enthalpy of formation, with. This table lists the.
Standard entropy wsmzaer
151 rows internationally agreed, internally consistent, values for the thermodynamic. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Values of the standard molar entropies of various substances at 298 k (25°c). ∗the standard entropy of the h+(aq) ion is.
Standard Enthalpies of Formation SchoolWorkHelper
The table below shows the standard enthalpy of formation, the standard gibbs free energy of. ∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of formation and absolute entropies of elements. 151 rows internationally agreed, internally consistent, values for the thermodynamic. This table lists the standard enthalpies (δh°), the free.
Standard entropy table Lasialabama
Values of the standard molar entropies of various substances at 298 k (25°c). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Definition and explanation of the terms standard state and standard enthalpy of formation, with. 151 rows internationally agreed, internally consistent, values for the thermodynamic. ∗the standard entropy of the h+(aq) ion is.
Standard entropy table filmqlero
∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of formation and absolute entropies of elements. 151 rows internationally agreed, internally consistent, values for the thermodynamic. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. Definition and explanation of the terms standard state and.
Solved Consider the following table of standard entropy
151 rows internationally agreed, internally consistent, values for the thermodynamic. Values of the standard molar entropies of various substances at 298 k (25°c). Definition and explanation of the terms standard state and standard enthalpy of formation, with. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. 100 rows standard heats and free energies of.
Solved 3. Use the tables of standard enthalpy of formation
151 rows internationally agreed, internally consistent, values for the thermodynamic. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. ∗the standard entropy of the h+(aq) ion is defined to be 0. 100 rows standard heats and free energies of.
Definition And Explanation Of The Terms Standard State And Standard Enthalpy Of Formation, With.
Values of the standard molar entropies of various substances at 298 k (25°c). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. 151 rows internationally agreed, internally consistent, values for the thermodynamic. ∗the standard entropy of the h+(aq) ion is defined to be 0.
The Table Below Shows The Standard Enthalpy Of Formation, The Standard Gibbs Free Energy Of.
100 rows standard heats and free energies of formation and absolute entropies of elements.