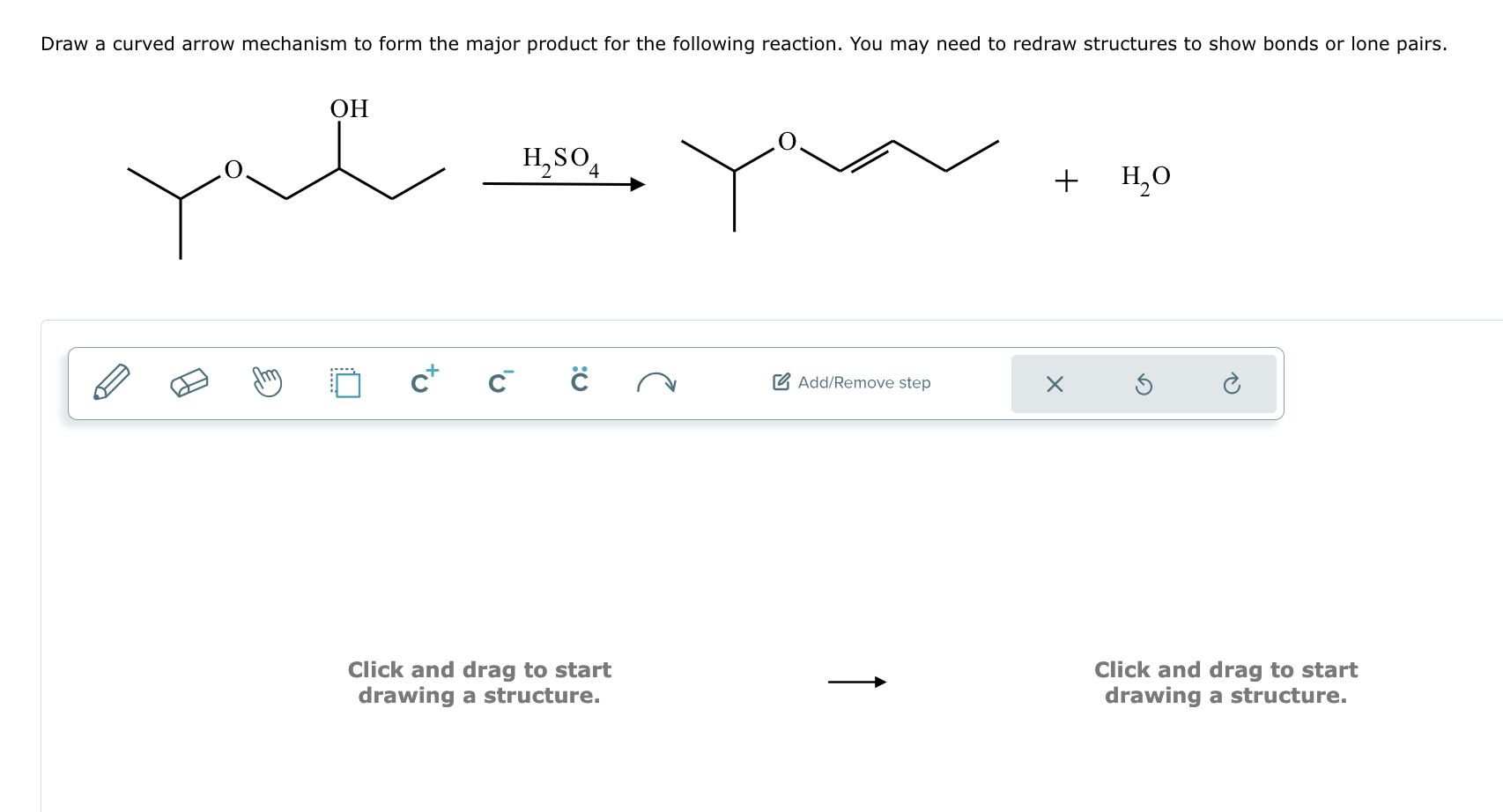
Two Or More Reactants Combine To Form One Product - A synthesis reaction occurs when two or more reactants combine to form a single. The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction. What is a synthesis reaction? A synthesis reaction is a reaction in which two or more.
A synthesis reaction is a reaction in which two or more. What is a synthesis reaction? A synthesis reaction occurs when two or more reactants combine to form a single. The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction.
A synthesis reaction is a reaction in which two or more. A process in which two or more chemicals combine to create a single new. What is a synthesis reaction? When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. The type of chemical reaction in which two reactants combine to form one new.
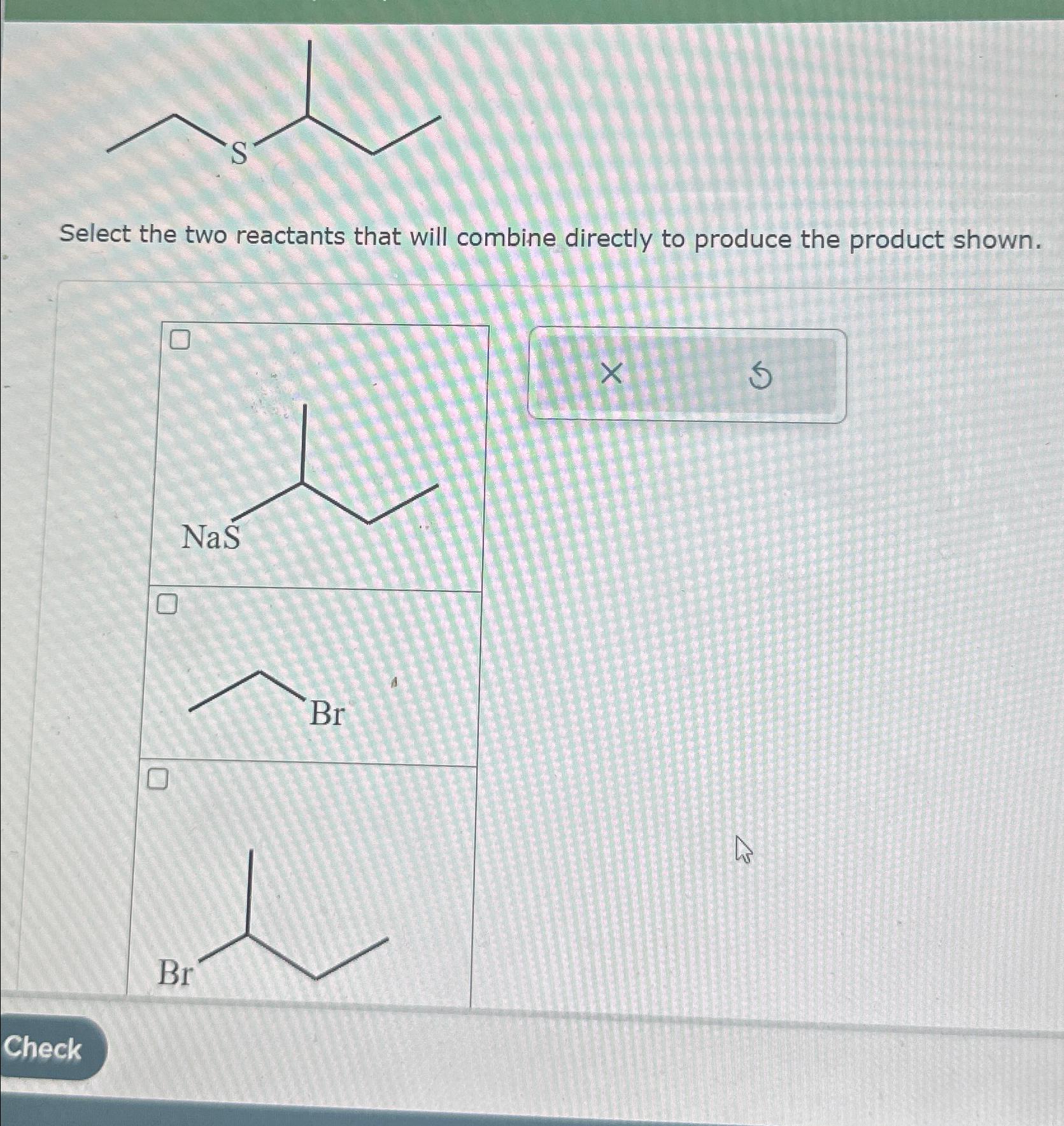
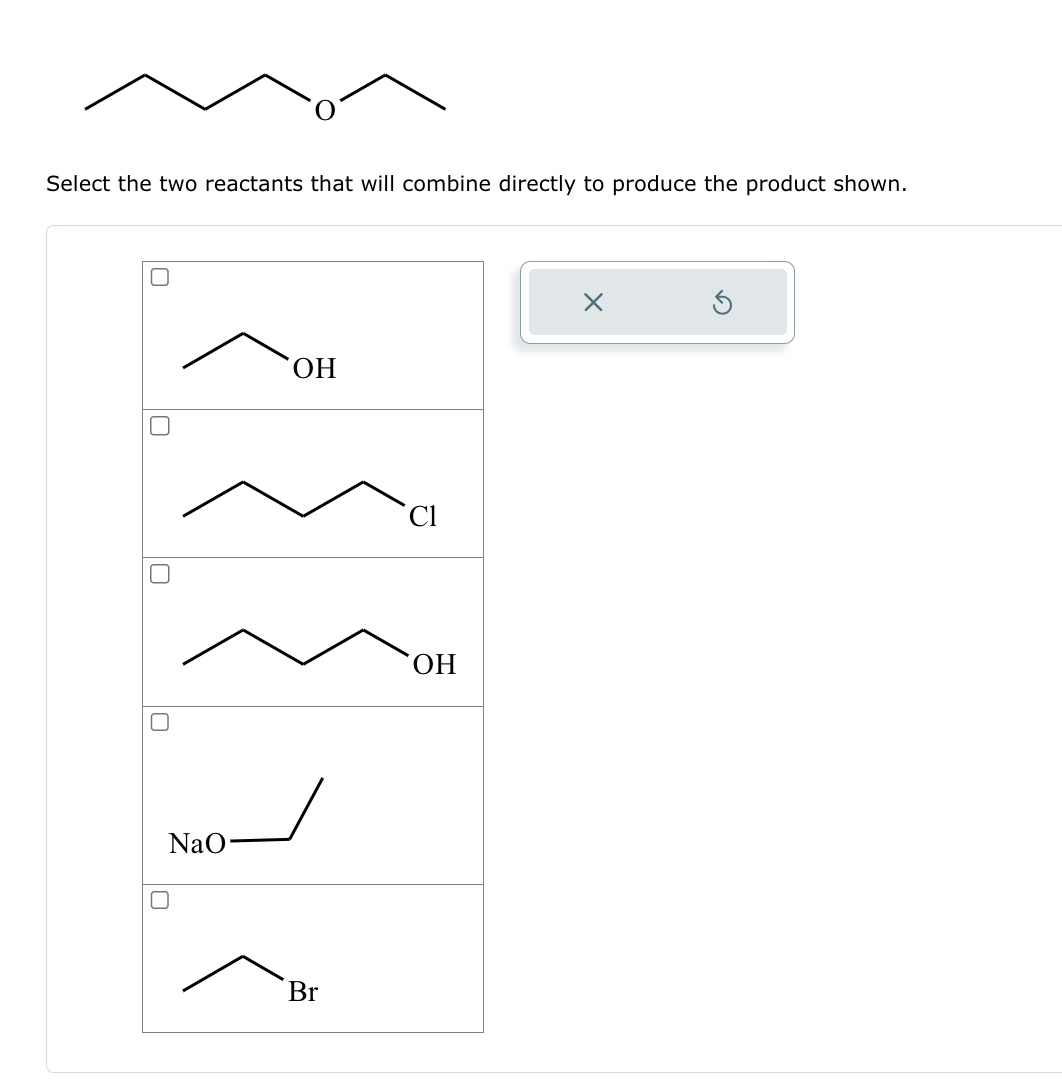
Solved Select the two reactants that will combine directly
A synthesis reaction occurs when two or more reactants combine to form a single. A synthesis reaction is a reaction in which two or more. When two or more simple reactants combine to form a new, more complex product, the reaction. What is a synthesis reaction? A process in which two or more chemicals combine to create a single new.
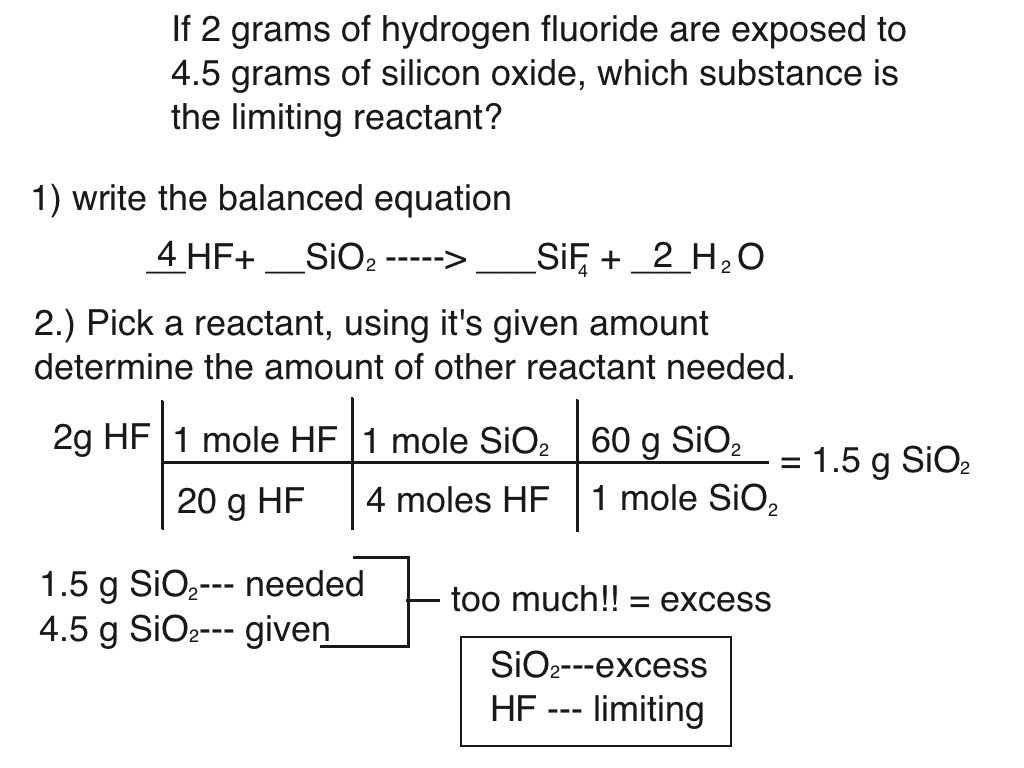
Limiting Reactants Chemistry 101
A process in which two or more chemicals combine to create a single new. A synthesis reaction is a reaction in which two or more. The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? When two or more simple reactants combine to form a new, more complex product, the reaction.
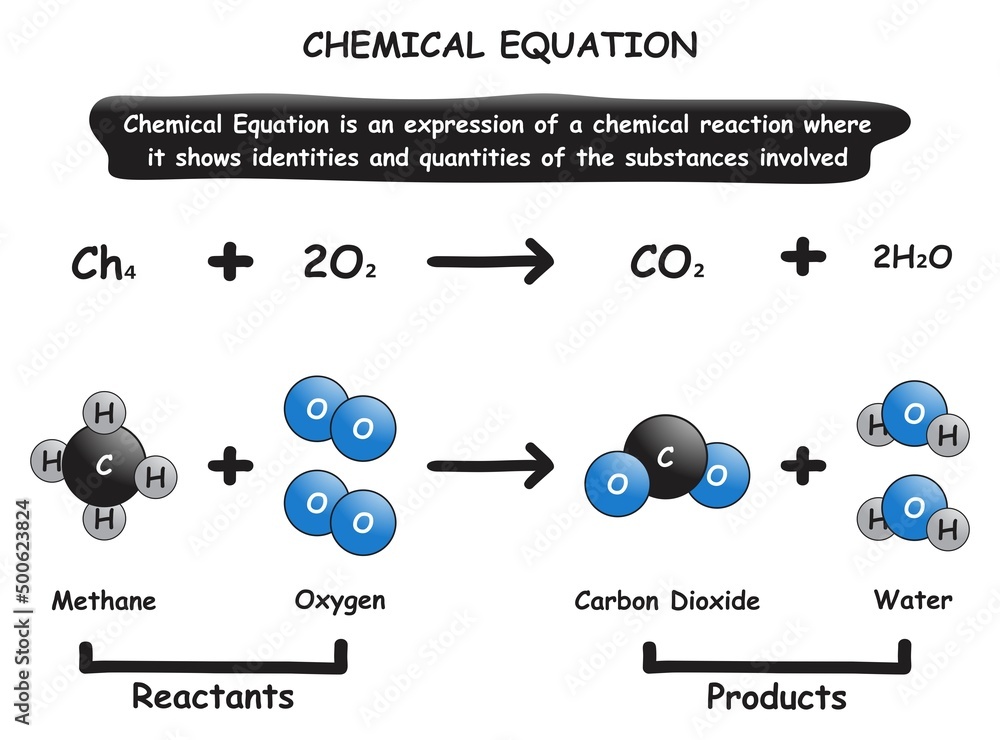
Chemical Equation Infographic diagram showing identities and quantities
When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. A process in which two or more chemicals combine to create a single new. What is a synthesis reaction? A synthesis reaction is a reaction in which two or more.
Solved Select the two reactants that will combine directly
A synthesis reaction is a reaction in which two or more. A process in which two or more chemicals combine to create a single new. The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? A synthesis reaction occurs when two or more reactants combine to form a single.
SOLVED Question 8 (5 points) Two reactants combine to form a product
What is a synthesis reaction? The type of chemical reaction in which two reactants combine to form one new. A synthesis reaction is a reaction in which two or more. A process in which two or more chemicals combine to create a single new. A synthesis reaction occurs when two or more reactants combine to form a single.
What Is The Chemical Equation For Photosynthesis Identify Reactants And
When two or more simple reactants combine to form a new, more complex product, the reaction. A process in which two or more chemicals combine to create a single new. The type of chemical reaction in which two reactants combine to form one new. A synthesis reaction is a reaction in which two or more. A synthesis reaction occurs when.
Synthesis Reactions occur when two of more reactants combine to
A process in which two or more chemicals combine to create a single new. A synthesis reaction is a reaction in which two or more. When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. What is a synthesis reaction?
Solved Select the two reactants that will combine directly
The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. A synthesis.
Reactants & Products of a Chemical Reaction Process & Examples
A synthesis reaction is a reaction in which two or more. The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? A process in which two or more chemicals combine to create a single new. A synthesis reaction occurs when two or more reactants combine to form a single.
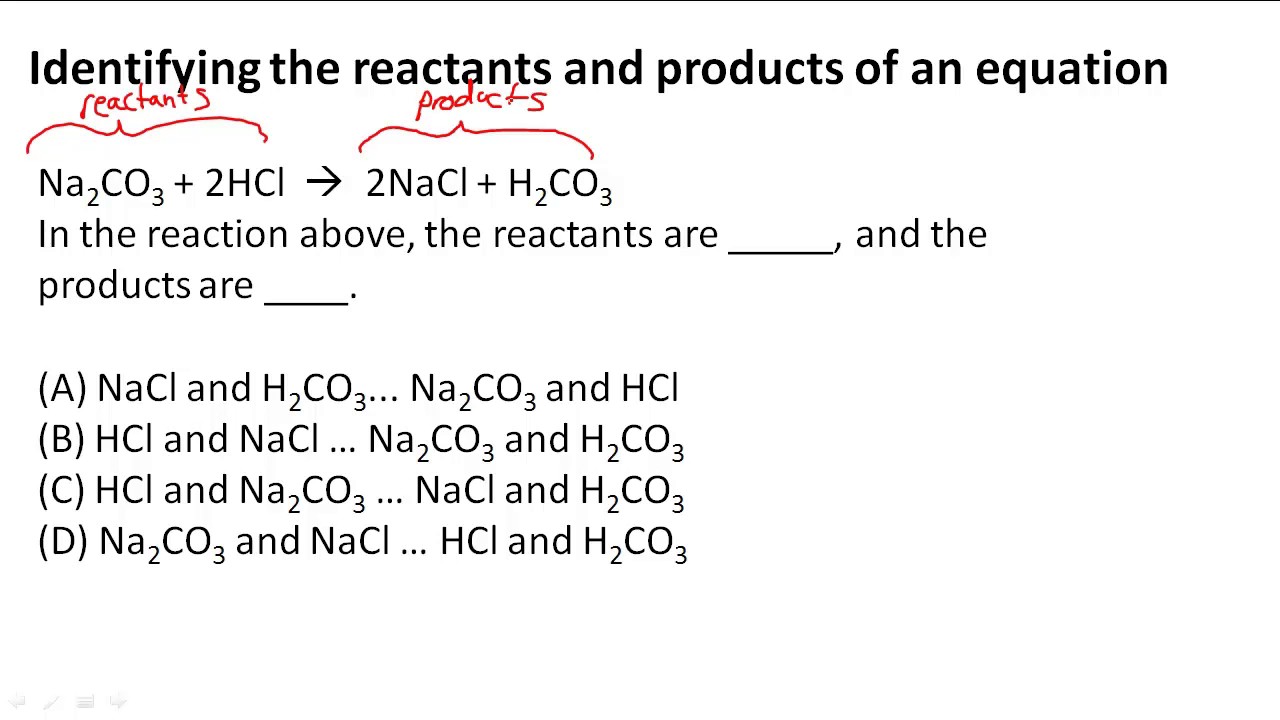
Solved Determine the reactants that produce the following products and
The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? A synthesis reaction is a reaction in which two or more. A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction.
A Synthesis Reaction Occurs When Two Or More Reactants Combine To Form A Single.
When two or more simple reactants combine to form a new, more complex product, the reaction. The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. A synthesis reaction is a reaction in which two or more.