Why Do Metals Form Cations - Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the. Metals form positive ions because they tend to lose electrons during chemical reactions,.
Atoms of metals tend to form cations because they generally have fewer than four electrons in. Metals form positive ions because they tend to lose electrons during chemical reactions,. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
Metals form positive ions because they tend to lose electrons during chemical reactions,. Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
Solved Question 1 Which metals form cations with varying
Atoms of metals tend to form cations because they generally have fewer than four electrons in. Metals form positive ions because they tend to lose electrons during chemical reactions,. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
Metals Tend to Form Cations or Anions IndiahasPeck
Even if the the metal has a smaller atomic radius due to occupying less shells, the. Atoms of metals tend to form cations because they generally have fewer than four electrons in. Metals form positive ions because they tend to lose electrons during chemical reactions,.
Do Metals Form Anions Or Cations
Atoms of metals tend to form cations because they generally have fewer than four electrons in. Metals form positive ions because they tend to lose electrons during chemical reactions,. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
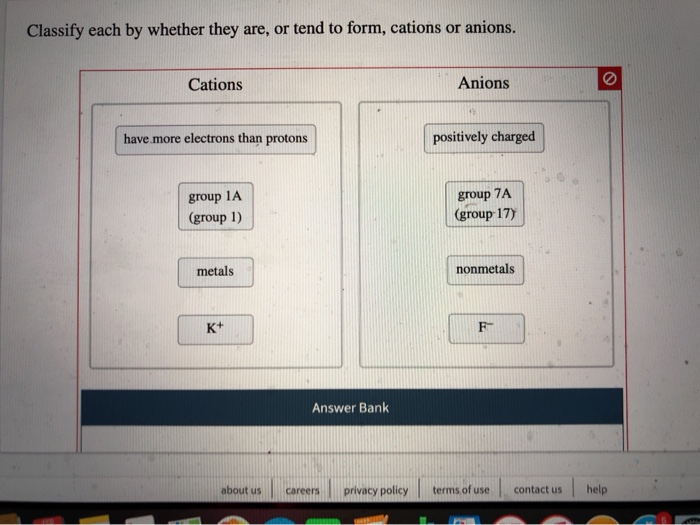
Cations and Anions Definitions, Examples, and Differences
Metals form positive ions because they tend to lose electrons during chemical reactions,. Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
Explain why almost all metals tend to form cations. Numerade
Even if the the metal has a smaller atomic radius due to occupying less shells, the. Atoms of metals tend to form cations because they generally have fewer than four electrons in. Metals form positive ions because they tend to lose electrons during chemical reactions,.
Solved Question 1 Which metals form cations with varying
Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the. Metals form positive ions because they tend to lose electrons during chemical reactions,.
Metals Tend to Form Cations or Anions IndiahasPeck
Metals form positive ions because they tend to lose electrons during chemical reactions,. Even if the the metal has a smaller atomic radius due to occupying less shells, the. Atoms of metals tend to form cations because they generally have fewer than four electrons in.
Which Metals Form Cations With Varying Positive Charges TerryhasYoder
Metals form positive ions because they tend to lose electrons during chemical reactions,. Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the.
Solved Question 1 Which metals form cations with varying
Atoms of metals tend to form cations because they generally have fewer than four electrons in. Even if the the metal has a smaller atomic radius due to occupying less shells, the. Metals form positive ions because they tend to lose electrons during chemical reactions,.
Do Metals Form Positive Or Negative Ions
Even if the the metal has a smaller atomic radius due to occupying less shells, the. Metals form positive ions because they tend to lose electrons during chemical reactions,. Atoms of metals tend to form cations because they generally have fewer than four electrons in.
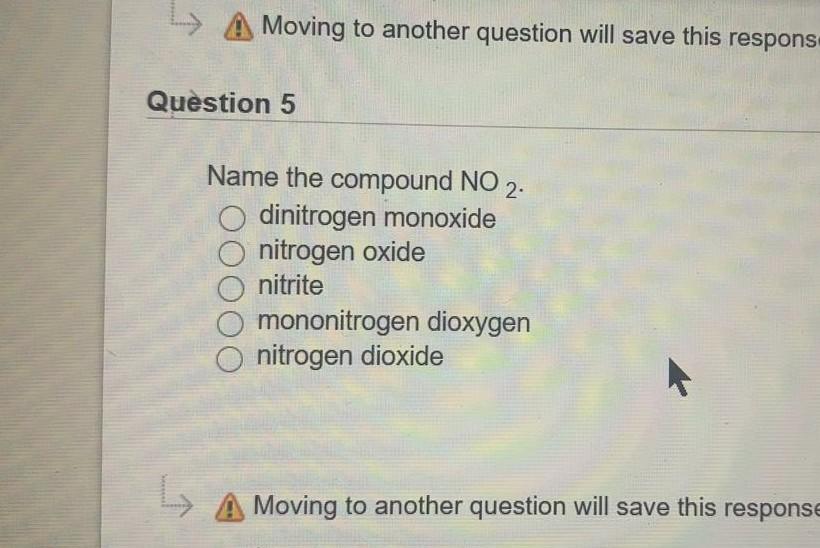
Atoms Of Metals Tend To Form Cations Because They Generally Have Fewer Than Four Electrons In.
Metals form positive ions because they tend to lose electrons during chemical reactions,. Even if the the metal has a smaller atomic radius due to occupying less shells, the.