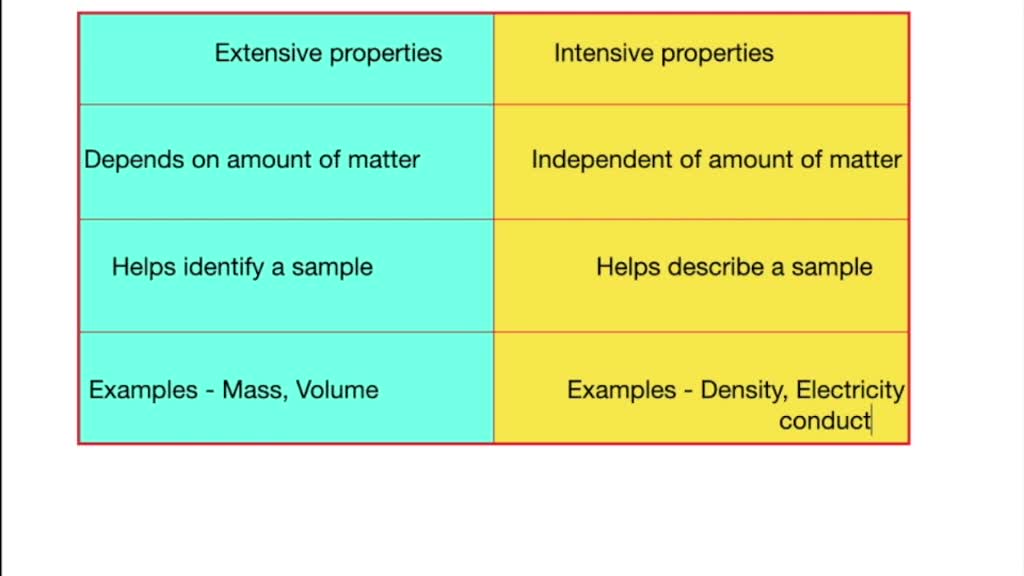
Differentiate Between Intensive And Extensive Properties - For example, in thermodynamics, the state of a. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. The distinction between intensive and extensive properties has some theoretical uses.
Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a.
The distinction between intensive and extensive properties has some theoretical uses. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. For example, in thermodynamics, the state of a.
Intensive/Extensive Properties Activity
Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses.
Intensive Properties vs. Extensive Properties — What’s the Difference?
For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like.
Extensive vs. Intensive Properties
For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like.
Difference Between Intensive and Extensive Properties of Matter
For example, in thermodynamics, the state of a. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. The distinction between intensive and extensive properties has some theoretical uses.
Difference Between Intensive and Extensive Properties Definition
Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses.
Difference Between Intensive and Extensive Properties of Matter
For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like.
Extensive vs Intensive Properties Difference and Comparison
The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like.
The Difference Between Intensive and Extensive Properties
For example, in thermodynamics, the state of a. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. The distinction between intensive and extensive properties has some theoretical uses.
What is the difference between extensive properties and intensive
Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like. The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a.
Intensive and Extensive Properties Worksheet
The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, the state of a. Extensive properties, such as mass and volume, vary with the amount of matter being considered, while intensive properties, like.
Extensive Properties, Such As Mass And Volume, Vary With The Amount Of Matter Being Considered, While Intensive Properties, Like.
For example, in thermodynamics, the state of a. The distinction between intensive and extensive properties has some theoretical uses.


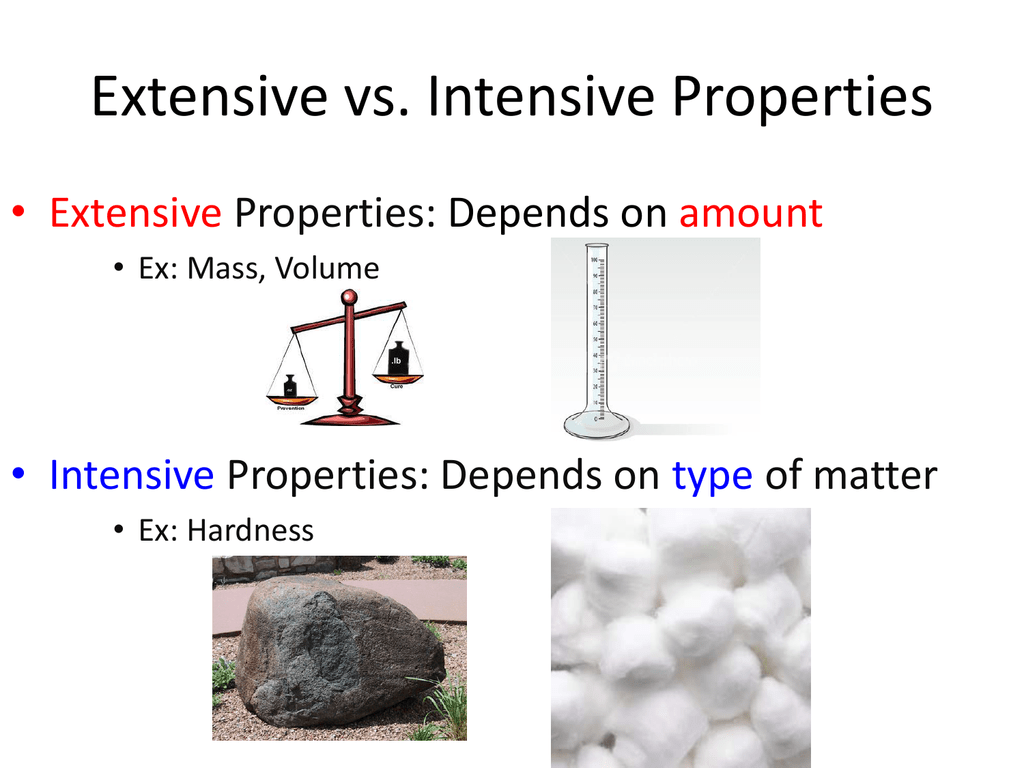

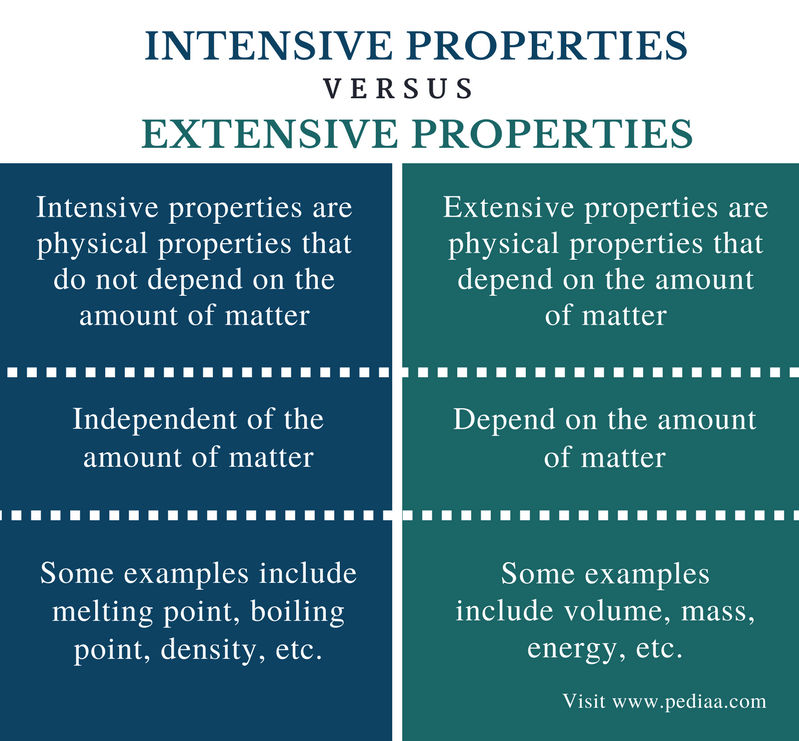


:max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png)